- Open access
- Published: 10 June 2024

New advances in the diagnosis and treatment of autism spectrum disorders
- Lei Qin 1 ,
- Haijiao Wang 2 ,
- Wenjing Ning 1 ,
- Mengmeng Cui 1 &
- Qian Wang 3
European Journal of Medical Research volume 29 , Article number: 322 ( 2024 ) Cite this article
1978 Accesses
1 Citations
Metrics details
Autism spectrum disorders (ASD) are a group of neurodevelopmental disorders that affect individuals' social interactions, communication skills, and behavioral patterns, with significant individual differences and complex etiology. This article reviews the definition and characteristics of ASD, epidemiological profile, early research and diagnostic history, etiological studies, advances in diagnostic methods, therapeutic approaches and intervention strategies, social and educational integration, and future research directions. The highly heritable nature of ASD, the role of environmental factors, genetic–environmental interactions, and the need for individualized, integrated, and technology-driven treatment strategies are emphasized. Also discussed is the interaction of social policy with ASD research and the outlook for future research and treatment, including the promise of precision medicine and emerging biotechnology applications. The paper points out that despite the remarkable progress that has been made, there are still many challenges to the comprehensive understanding and effective treatment of ASD, and interdisciplinary and cross-cultural research and global collaboration are needed to further deepen the understanding of ASD and improve the quality of life of patients.
Autism spectrum disorders (ASD) are a broad group of neurodevelopmental disorders that affect an individual's social interactions, communication skills, and behavioral patterns [ 1 , 2 ]. The characteristics of ASD vary significantly between individuals, from mild social impairments to severe communication and behavioral problems, a diversity that reflects the use of the term “spectrum” [ 3 ]. Although the exact causes of ASD are not fully understood, research suggests that both genetic and environmental factors play a key role in its development [ 4 ].
Characteristics of ASD
Difficulties in social interaction.
Individuals with ASD often exhibit significant difficulties in social interactions. These difficulties may include difficulty understanding the feelings and intentions of others, maintaining eye contact and facial expressions, and adapting to social norms and expectations. Individuals with ASD may experience challenges in establishing and maintaining friendships, they may not understand the two-way nature of social interactions, or they may feel uncomfortable sharing interests and activities [ 5 ].
Communication disorders
Communication deficits are another core feature of ASD. This may manifest itself in delays in language development, including delays in uttering first words or simple sentences. Some individuals with ASD may not use language to communicate at all. Even among individuals with ASD who have normal language skills, they may have difficulty using language in conversations to communicate thoughts, feelings, or needs. In addition, nonverbal communication, such as the understanding and use of body language and facial expressions, may also be affected [ 6 ].
Repetitive behaviors and interests
Individuals with ASD often display restricted, repetitive patterns of behavior and interests. These may include a strong fixation on specific topics or activities, repetitive body movements (e.g., rocking, clapping), and an overreliance on daily routines. These repetitive behaviors are sometimes seen as a way of self-soothing or as an attempt to control an environment that otherwise feels unpredictable and overwhelming to them [ 7 ].
Sensory sensitivity
Many individuals with ASD have abnormalities in sensory processing and may have very strong or delayed responses to sound, light, touch, taste or odor. For example, some individuals with ASD may find background noises in their everyday environment unusually harsh, or they may not notice pain or other bodily sensations [ 8 ].
Epidemiologic profile of ASD
According to the World Health Organization (WHO), the average prevalence of ASD among children globally is approximately 1% [ 9 ]. However, this figure varies significantly between regions and countries. For example, the Centers for Disease Control and Prevention (CDC) reports that the prevalence of ASD among 8-year-olds in the U.S. is 1 to 54. ASD is significantly more prevalent in males than females, at a ratio of approximately 4:1 [ 10 ]. This gender difference may reflect differences in genetic susceptibility and/or gender bias in the diagnostic process. Early diagnosis is key to improving developmental outcomes for children with ASD. Despite this, many children are not diagnosed by age 3. The CDC reports that most children are first evaluated for ASD by age 4, but diagnosis may occur later. Research suggests that ASD is highly heritable, but multiple genetic variants are associated with disease risk and environmental factors also play a role [ 11 ]. For example, there is an increased risk of ASD in preterm and low birth weight infants. Socioeconomic factors influence ASD diagnosis and treatment access. Families of lower socioeconomic status may face greater challenges, including barriers to accessing early intervention services, etc. ASD is a global public health problem, and its incidence, time to diagnosis, and treatment access are influenced by multiple factors [ 12 ]. Ongoing epidemiologic research and the advancement of a deeper understanding of ASD are critical to the development of effective prevention, diagnosis, and interventions.
Historical background
Early history of research and diagnosis of asd.
The concept of ASD was first clearly defined in the 1940s, when a group of children exhibiting extreme self-isolation and lack of responsiveness to the environment was first described by American psychiatrist Leo Kanner [ 13 ]. Almost simultaneously, Austrian child psychologist Hans Asperger described a similar but higher level of functioning in a condition that came to be known as Asperger’s syndrome [ 14 ]. These two independent studies laid the foundation for the modern understanding of ASD. For the first few decades, ASD was considered extremely rare and was often confused with schizophrenia. Due to a lack of in-depth understanding of ASD, early diagnostic criteria were unclear and treatment was largely limited to behavioral interventions and psychotherapy. Over time, researchers began to pay more attention to the genetic and neurobiological underpinnings of ASD, thus contributing to a more comprehensive understanding of this complex condition. Since the 1990s, the diagnosis of ASD has risen significantly, as diagnostic criteria have continued to be refined and public awareness has increased. This period has also witnessed an increased awareness of the importance of early diagnosis and intervention for ASD, which has led to significant improvements in the prognosis and quality of life for many children and adults with ASD [ 15 ].
Evolution of research paradigms
The research paradigm for ASD has undergone a remarkable evolution since the mid-twentieth century, a process that reflects a deepening of the understanding of ASD as well as advances in scientific research methods [ 16 ]. In the early stages, ASD research focused on behavioral observations and psychoanalysis, when ASD was often mistaken for an emotional disorder due to an indifferent mother. During this period, understanding of ASD was relatively limited and treatments focused primarily on psychotherapy and behavior modification. Into the second half of the twentieth century, with advances in genetics and neuroscience, researchers began to explore the biological basis of ASD. This marked a shift from a psychosocial to a biomedical model, and the focus of research gradually shifted to genetic factors and abnormalities in brain structure and function. Through a large number of family and twin studies, scientists found that ASD has a high genetic predisposition, while neuroimaging studies revealed the specificity of brain development in ASD patients. In the twenty-first century, with the application of bioinformatics and high-throughput gene sequencing technology, the study of ASD has entered a new stage [ 17 ]. Researchers have not only been able to identify specific genetic variants associated with ASD, but have also begun to explore the interaction between environmental factors and genetic susceptibility. In addition, the adoption of interdisciplinary research approaches, such as combining neuroscience, genetics, psychology, and computational modeling, has provided new perspectives for understanding the complexity of ASD.
Recently, the concepts of precision medicine and personalized treatment strategies have been introduced to the study of ASD, aiming to develop customized intervention programs based on each patient’s genetic background and symptom profile. With advances in technology and improved methods of data analysis, future research on ASD is expected to reveal more knowledge about its pathomechanisms and provide more effective support and treatment for patients with ASD.
Etiologic studies
Genetic factors, monogenic genetic cases.
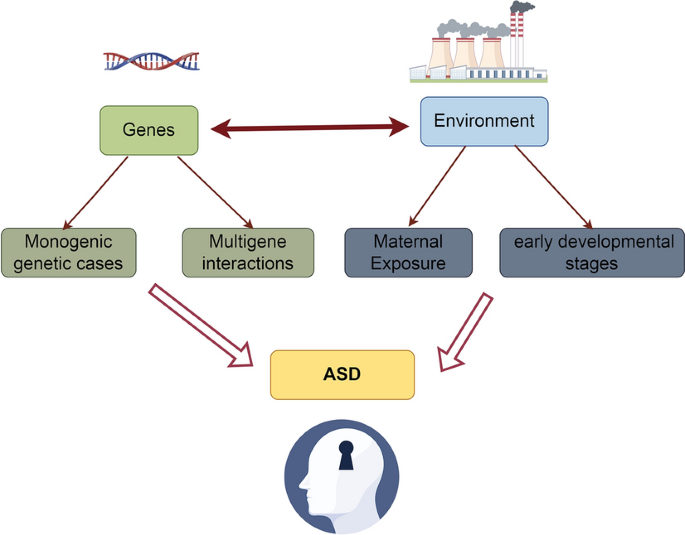
The etiology of ASD is multifactorial, involving a complex interaction of genetic and environmental factors. Although most cases of ASD are thought to be the result of polygenic interactions, there are some cases that are directly associated with variations in a single gene, and these are referred to as monogenic genetic cases. Monogenic genetic cases provide an important window into understanding the genetic basis of ASD, although they represent a relatively small proportion of all ASD cases [ 18 ]. A number of specific genetic syndromes, such as fragile X syndrome, tuberous sclerosis, 15q11-q13 duplication syndrome, and Rett syndrome, have been found to be associated with a higher risk of ASD. These conditions, often caused by mutations or abnormalities in a single gene, can lead to significant differences in brain development and function, thereby increasing the probability of an ASD phenotype. Fragile X syndrome is one of the most common forms of inherited intellectual disability and the single-gene disorder known to be most strongly associated with ASD. It is caused by a repeat expansion on the FMR1 gene [ 19 ]. Tuberous sclerosis (TSC) is an inherited disorder that affects multiple systems and is caused by mutations in the TSC1 or TSC2 genes, and the prevalence of ASD is higher in patients with TSC. 15q11-q13 duplication syndrome (Dupuy 15q syndrome) involves a region of chromosome 15, the duplication of which is associated with an increased risk of ASD [ 20 ]. Rett syndrome, which predominantly affects females, is caused by mutations in the MECP2 gene, and patients often exhibit some of the features of ASD, such as impaired social interactions [ 21 ]. The association of these classical candidate genes with ASD is summarized in Table 1 .
The discovery of these monogenic genetic cases is not only crucial for understanding the genetic mechanisms of ASD, but also potentially valuable for the development of interventional and therapeutic strategies targeting specific genetic variants. However, even in these cases, the expression of the genetic variants showed a degree of heterogeneity, suggesting that the diversity of phenotypic features and clinical manifestations, even in monogenic genetic cases, may be influenced by other genetic and environmental factors. Therefore, an in-depth study of these conditions will not only improve our understanding of the genetic basis of ASD, but also provide clues for the development of more personalized therapeutic strategies.
Multigene interactions
The development of ASD is widely recognized as a result of the interaction of genetic and environmental factors, with polygenic interactions occupying a central position in the genetic background of the disease. Unlike monogenic cases, polygenic interactions involve variants or polymorphisms in multiple genes that together increase the risk of ASD. These genetic variants may contribute a smaller effect in each individual, but when acting together they can significantly increase the probability of ASD development [ 30 ]. Current research suggests that no single gene can explain all cases of ASD. Instead, hundreds of genetic loci have been identified that are associated with an increased risk of ASD. These genes are often involved in key processes such as brain development, neuronal signaling, and intercellular communication, suggesting that ASD involves extensive regulation of brain function and structure. The complexity of multigene interactions means that genetic studies of ASD require large-scale genomic data and sophisticated statistical methods to reveal those genomic variants that increase risk.
Meta-analyses of large-sample genome-wide association studies (GWAS) have identified several consistently replicated ASD risk gene loci, such as those in the chromosomal regions 3p21, 5p14, 7q35, and 20p12. These loci contain genes like CNTN4, CNTNAP2, and NRXN1, which play crucial roles in neurodevelopment and synaptic function, particularly in processes such as synaptic adhesion and neurotransmission. These findings provide a more robust understanding of the genetic architecture of ASD and highlight the importance of integrating genetic findings with functional studies to advance our understanding of the disorder. They also have implications for future research, such as the development of personalized diagnostic and therapeutic strategies based on an individual's genetic profile. Through genome-wide association studies (GWAS) and other genomic approaches, scientists are gradually unraveling the genetic landscape of this complex disease. Understanding the impact of multiple gene interactions on ASD not only helps us understand its genetic basis, but also opens up the possibility of developing personalized treatment and intervention strategies [ 31 ].
Environmental factors
Maternal exposure.
Exposure during pregnancy refers to a mother’s exposure to specific environmental factors or substances during fetal development, which may increase the child's risk of developing ASD in the future. These exposures include certain prescription medications (e.g., anti-seizure medications and opioids), environmental pollutants (e.g., heavy metals and air pollutants), infections (e.g., rubella and influenza viruses), and poor nutrition or deficiencies in specific nutrients (e.g., folic acid). These factors may increase the risk of ASD by affecting fetal brain development and the maturation process of the nervous system. Understanding the effects of exposure during pregnancy can help to take preventive measures to reduce the incidence of ASDs [ 32 ].
Effects of early developmental stages
The early developmental stages of ASD are influenced by a variety of factors that include genetic predisposition, environmental exposures, and early life experiences. During a child's early development, the brain experiences rapid growth and the formation of neural networks. Any disruption during this critical period may interfere with the proper development of brain structure and function, thereby increasing the risk of ASD. For example, very early lack of social interaction, delayed language development or abnormal sensory processing may be early signs of ASD. These developmental abnormalities reflect difficulties in the brain’s nervous system in processing information, making connections and adapting to environmental changes. Early identification and intervention are essential to promote optimal development in children with ASD [ 33 ].
Genetic–environmental interactions
The genetic–environmental interactions are summarized in Fig. 1 . ASD develops as a result of the interaction between genetic and environmental factors, and this interaction reflects the complexity of the combination of genetic background and external environmental factors that influence ASD risk. Specifically, certain genetic susceptibilities may be activated in response to environmental triggers, leading to the development of ASD. For example, genetic variants may make individuals more sensitive to certain environmental exposures (e.g., substance use during pregnancy, environmental pollutants, or maternal nutritional status), which together may increase the risk of ASD by acting on key brain developmental stages [ 34 ]. This complex genetic–environmental interaction underscores the need to understand multifactorial etiological models of ASD and the importance of developing personalized intervention strategies.
Advances in diagnostic methods
Traditional diagnostic methods.
Traditional diagnostic methods for ASD rely heavily on detailed assessments of behavior and developmental history. These assessments are usually conducted by specialized health care providers such as pediatricians, neuropsychologists, or psychiatrists. The diagnostic process encompasses direct observation of the child as well as in-depth interviews with parents or caregivers to gather information about the child's social interactions, communication skills, and behavioral patterns [ 35 ]. Diagnostic tools include, but are not limited to, the Childhood Autism Rating Scale (CARS), the Autism Diagnostic Observation Scale (ADOS), and the Autism Diagnostic Interview-Revised (ADI-R). These tools are designed to identify core symptoms of ASD, such as social communication deficits and repetitive behaviors or interests. In addition, the doctor may perform a series of developmental or cognitive assessments to rule out other conditions that may explain the child’s behavior, such as language disorders or other neurodevelopmental disorders [ 36 ]. While these traditional diagnostic methods are highly effective in recognizing ASD, they rely on subjective assessments and the experience of the professional, and therefore may have some degree of variability. In recent years, with a deeper understanding of ASDs, new diagnostic techniques and methods are being developed and adopted to improve diagnostic accuracy and efficiency.
Latest diagnostic techniques and tools
Genetic testing.
Genetic testing for ASD is a method of identifying risks associated with ASD by analyzing genetic variants in an individual's DNA. This testing looks for specific genetic variants that have been linked by scientific research to the development of ASD. Although the genetic background of ASD is extremely complex, involving multiple genes and the interaction of genes with environmental factors, variants in specific genes have been identified as having a significant impact on ASD risk [ 37 ]. For example, variants in the SHANK3 gene are associated with Phelan–McDermid syndrome, and patients with this syndrome often exhibit ASD features. Variants in the FMR1 gene are responsible for fragile X syndrome, which is the most common single-gene cause of ASD known to be associated with ASD. Mutations in the MECP2 gene have been associated with Rett syndrome, and patients with Rett syndrome often exhibit ASD condition. In addition, variants in the NRXN1 and NLGN3/4 genes have been found to increase the risk of ASD [ 38 ]. Genetic testing can help provide more precise diagnostic information, and in those cases of ASD where the cause is unknown, it may even reveal the underlying genetic cause. This will not only help to understand the genetic mechanisms of ASD, but also provide more targeted intervention and support strategies for patients and families.
Neuroimaging
Neuroimaging techniques in the study of ASD provide a non-invasive way to explore changes in brain structure and function, helping scientists better understand the biological basis of ASD. These techniques include functional magnetic resonance imaging (fMRI), structural magnetic resonance imaging (sMRI), diffusion tensor imaging (DTI), and positron emission tomography (PET). Through these neuroimaging techniques, researchers are able to observe structural and functional differences in specific regions and networks of the brain in individuals with ASD [ 39 ]. For example, fMRI can reveal patterns of brain activity when performing specific tasks, helping to understand the impairments in social, language, and cognitive functioning in individuals with ASD. dTI focuses on the microstructure of the brain’s white matter, revealing the connections of bundles of nerve fibers, which can help to study neural connectivity issues in ASD. PET scans, on the other hand, are able to assess the activity of specific chemicals in the brain, providing clues to study the neurochemical basis of ASD [ 40 ]. With these advanced neuroimaging techniques, researchers will not only be able to delve deeper into the neurodevelopmental abnormalities of ASD, but also identify possible novel therapeutic targets that can provide a scientific basis for developing more effective interventions. However, while these techniques provide valuable perspectives in understanding ASD, a complete understanding of the complexity of the brain remains a challenge for future research.
Early screening methods
Recently, the field of early screening for ASD has witnessed the application of a number of innovative techniques designed to improve the accuracy and convenience of screening. One notable new approach is the use of artificial intelligence (AI) and machine learning techniques to analyze children's behavioral videos and biomarkers. By training algorithms to recognize specific behavioral patterns and physiological signals associated with ASD, these technologies can help physicians and researchers identify potential ASD symptoms earlier [ 41 ]. Another area of innovation is eye-tracking technology, which assesses children’s social and cognitive development by analyzing their eye movement patterns when viewing pictures or videos. Studies have shown that the eye movement patterns of children with ASD while viewing social scenes differ from those of typically developing children, providing a non-invasive window for early screening [ 42 ]. The application of these state-of-the-art technologies not only improves the efficiency and accessibility of early screening, but also provides new perspectives for understanding the complexity and individual differences in ASD [ 43 ]. Although these approaches are still in the research and development stage, they demonstrate the great potential of utilizing technological advances to improve the process of ASD screening and diagnosis. With further validation and refinement of these techniques, it is expected that they will make a significant contribution to the early identification and intervention of ASD in the future.
Treatment approaches and intervention strategies
Behavioral and educational interventions, applied behavior analysis (aba).
Applied behavior analysis (ABA) is an intervention approach based on the principles of behavioral psychology that is widely used in the treatment of children with autism spectrum disorders (ASD). ABA works to understand and improve specific behaviors, particularly to enhance social, communication, academic skills, and daily living skills, while reducing maladaptive behaviors. It helps individuals learn new skills and behaviors by systematically applying reinforcement strategies that encourage and reward desired behaviors [ 44 ]. ABA therapy is highly individualized and customized to each child’s specific needs and abilities. Treatment planning begins with a detailed behavioral assessment to identify target behaviors and intervention strategies. Learned behaviors are then reinforced and cemented through one-on-one teaching sessions using positive reinforcement. ABA also emphasizes the importance of data, which is collected and analyzed on an ongoing basis by the therapist to monitor progress and adjust the treatment plan as necessary [ 45 ]. Research has shown that ABA is an effective way to improve social interactions, communication skills, and learning in children with ASD. Through early and consistent intervention, ABA can significantly improve the independence and overall quality of life of children with ASD. Although ABA treatment requires a commitment of time and resources, the long-term benefits it brings to children with ASD and their families are immeasurable.
Social skills training
Social skills training (SST) for children with autism spectrum disorders (ASD) is an intervention designed to improve their ability to interact socially in everyday life. This training focuses on teaching children with ASD the ability to understand social cues, establish effective communication skills, and develop friendships. Through SST, children learn how to recognize and interpret other people's facial expressions, body language, and social etiquette, which are essential for building positive relationships [ 46 ]. Social skills training typically includes a series of structured instructional activities such as role-playing, social stories, interactive group exercises, and peer modeling. These activities are designed to provide practice in real-world social situations in a supportive and interactive manner, helping children with ASD learn and practice new skills in a safe environment [ 47 ]. In addition, SST can include teaching emotion management and conflict resolution skills to help children with ASD better understand and express their emotions and cope with challenges in social interactions. Through regular and consistent practice, children with ASD can improve their self-confidence, increase their social engagement, and ultimately improve their social competence and quality of life. SST has been shown to be significantly effective in enhancing social adjustment and interpersonal interactions in children with ASD [ 48 ].
Medical treatment
While there is no cure for ASD, certain medications can be used to manage specific symptoms associated with ASD, such as behavioral problems, attention deficits, anxiety, and mood swings that are common in individuals with autism. Medication is often used as part of a comprehensive intervention program designed to improve the quality of life and daily functioning of the patient [ 49 ]. Medications commonly used for ASD symptom management include antipsychotics, antidepressants, stimulants, and anxiolytics. For example, two antipsychotics, risperidone and aripiprazole, have been approved by the FDA for the treatment of stereotypic and aggressive behavior in children and adolescents with ASD. In addition, selective serotonin reuptake inhibitors (SSRIs) may be helpful in managing anxiety and depressive symptoms in individuals with ASD.
Importantly, medication needs to be closely monitored by a physician to ensure the effectiveness and safety of the medications, as they may have side effects. We have summarized the research evidence on the efficacy and safety of commonly used medications in ASD, including antipsychotics for treating irritability and aggression, antidepressants for co-occurring anxiety and depression, and other medications such as stimulants and melatonin. While these medications can be helpful in managing specific symptoms, they also carry potential side effects and risks, such as weight gain, metabolic disturbances, and behavioral activation. Therefore, a thorough diagnostic evaluation, individualized treatment planning, close monitoring, and regular follow-up are essential when considering pharmacotherapy for individuals with ASD. The decision to medicate should be based on an individualized assessment that takes into account the patient’s specific needs, the severity of symptoms, and possible side effects. At the same time, pharmacological treatments are often used in combination with non-pharmacological treatments such as behavioral interventions and educational support to achieve optimal therapeutic outcomes [ 50 ].
Biofeedback and neuromodulation
Biofeedback and neuromodulation are innovative approaches that have been explored in recent years in the treatment of ASD, aiming to reduce ASD symptoms by improving brain function. Biofeedback techniques enable individuals to learn how to control physiological processes that are not normally under conscious control, such as heart rate, muscle tension, and brainwave activity. Through real-time feedback, patients can learn how to regulate their physiology, resulting in improved concentration, reduced anxiety, and improved emotional regulation. Neuromodulation, specifically transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS), affects neural activity in the brain through external stimulation. tMS utilizes a magnetic field to affect neuronal activity in specific areas of the brain, while tDCS modulates neuronal excitability by applying a weak electrical current. These methods have been studied for improving social communication skills and reducing stereotypical behaviors in people with ASD [ 51 ].
Biofeedback helps individuals develop self-regulation skills by providing real-time feedback on physiological states, while neuromodulation techniques like TMS and tDCS modulate cortical excitability and neural plasticity in aberrant circuits implicated in ASD. Current research suggests potential benefits of these techniques in improving emotional regulation, social functioning, and cognitive performance, but mixed results highlight the need for larger, well-controlled trials to validate efficacy, safety, and optimal protocols. Despite challenges, these techniques show promise as adjunctive therapies in the comprehensive management of ASD, warranting further research to guide their translation into clinical practice. Although biofeedback and neuromodulation show potential in the treatment of ASD, research on these techniques is currently in its infancy. More clinical trials and studies are needed to evaluate their effectiveness, safety, and long-term effects and to determine which patients may benefit from these interventions. Nevertheless, as non-pharmacologic treatments, they offer promising complementary options to the comprehensive treatment of ASD.
Emerging intervention approaches
Technology-assisted interventions.
Technology-assisted interventions have become an important development in the field of ASD treatment in recent years, providing new ways for children with ASD to learn and communicate. These interventions utilize computers, tablets, smartphone apps, and virtual reality technology to design a range of interactive learning tools and games designed to improve social skills, communication, and cognitive functioning in children with ASD [ 52 ]. A key advantage of technology-assisted interventions is their ability to provide highly personalized learning experiences. Software and applications can be adapted to a child's specific needs and interests, ensuring that learning content is both engaging and appropriate to the individual's developmental level. In addition, the feedback provided by technology is often immediate and consistent, helping children with ASD to better understand and process information. The use of virtual reality technology, by simulating social situations, provides a safe and controlled environment for children with ASD to practice social interaction and problem-solving skills, which is often difficult to achieve in traditional educational and therapeutic settings [ 53 ]. Although technology-assisted interventions have demonstrated great potential, research on their long-term effects and optimal implementation is still ongoing. To maximize the benefits of these tools, it is often recommended that technology-assisted interventions be used in conjunction with other therapeutic approaches to provide a comprehensive intervention program.
Diet and nutrition interventions
Dietary and nutritional interventions have received increasing attention in the treatment of ASD, based on the observed potential link between nutritional imbalances and ASD symptoms. This intervention approach aims to improve the behavioral performance and overall health of children with ASD by optimizing their diet. Specific strategies include restricting certain foods that may exacerbate symptoms, such as gluten and lactose, as well as increasing intake of foods rich in essential nutrients to support brain development and function [ 54 ]. Several studies support the potential benefits of specific dietary interventions, such as implementing a gluten-free lactose-free (GFCF) diet, which may help improve behavioral and digestive symptoms in some children with ASD. In addition, supplementation with omega-3 fatty acids, vitamins, and minerals (e.g., magnesium and zinc) have been proposed as potentially beneficial strategies to support neurologic health and alleviate ASD-related symptoms [ 55 ]. However, the effectiveness of dietary and nutritional interventions may vary by individual and more scientific research is needed to gain a deeper understanding of their long-term effects on children with ASD. Before implementing any dietary intervention, it is recommended to consult with a physician or nutritional expert to ensure that the individual needs of the child are met and to avoid malnutrition. In combination, dietary and nutritional interventions can be used as part of a comprehensive treatment plan for ASD, complementing traditional behavioral and educational interventions.
Social and educational integration
Educational integration of children with asd.
Educational integration of children with ASD is an inclusive educational practice that seeks to integrate children with ASD into the mainstream educational system to learn and grow with their typically developing peers. This integration model emphasizes individualized learning plans and adaptive teaching strategies to meet the unique needs of children with ASD while promoting their social inclusion and emotional development. Through educational integration, children with ASD are provided with opportunities to interact with other children, which is essential for them to learn social skills, enhance their communication abilities, and improve their ability to adapt to society. To support the successful integration of children with ASD, schools often provide special education services such as speech and language therapy, occupational therapy, and behavioral interventions, which take place in classroom settings to ensure their academic and social progress. Educational inclusion is not only beneficial for children with ASD, but it also helps to foster a sense of inclusion and diversity among their peers. By learning and playing together, all children learn to respect and understand differences, laying the foundation for a more inclusive society. However, effective integrated education requires close collaboration among teachers, parents and professionals, as well as the availability of appropriate resources and support systems [ 56 ].
Social integration and employment of adults with ASD
The social integration and employment of adults with ASD is a current focus of attention in ASD research and social services. For many adults with ASD, social integration challenges include establishing stable relationships, participating in community activities, and finding and keeping a job. Although adults with ASD may have unique skills and interests in specific areas, social communication deficits and fixed patterns of behavior may make it difficult for them in traditional work settings. In recent years, more and more organizations and businesses have begun to recognize the value of diversity and inclusion and are working to create work environments that are better suited for adults with ASD. This includes providing flexible work arrangements, clear communication guidelines, and individualized support measures such as workplace co-worker support and professional career counseling. In addition, social service programs and non-profit organizations offer training and job readiness programs specifically designed for adults with ASD to help them develop necessary vocational skills and social competencies. Through these efforts, adults with ASD will not only be able to find jobs that meet their interests and abilities, but also find a place for themselves in society, enhancing their independence and life satisfaction. However, the realization of this goal requires sustained social awareness-raising and the construction of an ASD-friendly environment [ 57 ].
Future research directions
Application of precision medicine in asd treatment.
The application of precision medicine in the treatment of ASD represents a paradigm of a personalized treatment strategy that aims to tailor the treatment plan to each patient's genetic information, biomarkers, history of environmental exposure, and lifestyle factors. The philosophy behind this approach is that, although ASD is classified as a spectrum, each patient's etiology, symptoms, and their severity are different, and therefore treatment should be highly individualized [ 58 , 59 ]. By fully sequencing a patient's genome, scientists and physicians can identify specific genetic variants that may affect ASD symptoms, allowing them to develop targeted treatments. For example, if a particular ASD patient's symptoms are linked to an abnormality in a specific metabolic pathway, that pathway could be modulated through dietary adjustments, nutritional supplements, or specific medications with a view to improving symptoms. In addition, precision medicine involves the consideration of environmental factors and personal behavior to ensure that treatment options are not only scientifically effective, but also appropriate to the patient's lifestyle. Although precision medicine is still in its early stages in the field of ASD, it offers great potential for delivering more personalized and effective treatment regimens, which are expected to significantly improve the quality of life of people with ASD [ 60 ].
Prospects for emerging biotechnologies
Emerging biotechnologies in the field of ASD, such as gene editing, stem cell therapies, and biomarker development, are opening up new possibilities for treating and understanding ASD. Gene editing technologies, particularly the CRISPR-Cas9 system, provide researchers with the means to precisely modify genetic variants associated with ASD, promising to reveal how specific genetic variants affect brain development and function, thereby providing clues for the development of targeted therapies [ 61 ]. Stem cell therapies utilize a patient's own induced pluripotent stem cells (iPSCs) to study the pathomechanisms of ASD by mimicking the neurodevelopmental process in vitro, as well as exploring potential cellular alternative treatments. In addition, the discovery of biomarkers facilitates early diagnosis and monitoring of disease progression, making personalized treatment possible [ 62 ]. In addition, induced pluripotent stem cell (iPSC)-derived brain organoids from ASD patients have emerged as a powerful tool for studying the neurodevelopmental abnormalities associated with ASD. These 3D, self-organizing models recapitulate key features of human brain development in vitro, allowing researchers to investigate the cellular and molecular mechanisms underlying ASD pathogenesis. By comparing brain organoids derived from ASD patients with those from healthy controls, researchers can identify alterations in neuronal differentiation, migration, and connectivity that may contribute to the development of ASD. Moreover, patient-derived brain organoids provide a personalized platform for drug screening and testing, enabling the identification of targeted therapies that can be tailored to an individual's genetic background. This approach has the potential to revolutionize the development of precision medicine strategies for ASD, by providing a more accurate and relevant model system for investigating disease mechanisms and testing novel therapeutic interventions. As the field continues to advance, iPSC-derived brain organoids are expected to play an increasingly important role in unraveling the complex etiology of ASD and guiding the development of personalized treatment strategies [ 63 ]. The development of these technologies has not only improved our understanding of the complex etiology of ASD, but also provided more precise and effective treatment options for ASD patients. Although most of these emerging biotechnologies are still in the research phase, they bring hope and anticipation for the future of ASD treatment and management. As research progresses and technology matures, it is expected that these innovative approaches will bring substantial benefits to individuals with ASD and their families.
Interaction between social policy and ASD research
The interaction between social policy and ASD research is key to achieving better social inclusion and quality of life for individuals with ASD and their families. Effective social policies can provide the necessary financial support and legal framework for ASD research, promoting a deeper understanding of ASD and the development of new treatments. For example, policies can promote collaboration in interdisciplinary research, encourage the use of innovative technologies and methods, and support long-term follow-up studies. In addition, social policies play a crucial role in ensuring that ASD research results are translated into practical applications and that education, employment, and social services are provided to individuals with ASD. Through the development of inclusive education policies, employment assistance programs, and the provision of integrated social services, policies can help individuals with ASD realize their potential and better integrate into society. At the same time, advances in ASD research also provide a scientific basis for the development of more targeted and effective social policies, helping policymakers understand the needs of individuals with ASD and develop more precise support measures. Thus, there is a close interplay between social policy and ASD research, which together have contributed to the advancement of the understanding of ASD and coping strategies.
Limitations of the current research
Although significant progress has been made in ASD research, a number of key limitations remain. First, the etiology of ASD is extremely complex, involving genetic and environmental factors and their interactions, making it extremely challenging to identify specific etiologies and develop targeted treatment strategies. Second, the heterogeneity of ASD is reflected in the extreme variability of symptoms among patients, which makes it difficult to develop uniform diagnostic criteria and treatment approaches. In addition, most studies have focused on children, and adult patients with ASD have been relatively understudied, which limits the understanding of the full lifespan of ASD. In terms of research methodology, most current ASD research relies on small, short-term studies, which may affect the broad applicability of results and the assessment of long-term effectiveness. In addition, although advances in technology have provided new tools for ASD diagnosis and intervention, the popularization and application of these technologies still face economic and resource constraints. Finally, ASD research is unequal across the globe, with far more research conducted in resource-rich countries and regions than in resource-limited areas. This imbalance limits a comprehensive understanding of ASD in different cultural and social contexts. Therefore, to overcome these limitations, more interdisciplinary, cross-cultural, and long-term research, as well as global collaborations, are needed to deepen the understanding of ASD and improve the quality of life of individuals with ASD.
Perspectives on future research
The outlook for future prevention and treatment of ASD points in a more individualized, integrated, and technology-driven direction. With a deeper understanding of the genetic and environmental factors of ASD, it is expected that more targeted interventions and therapeutic strategies will be developed that will be based on an individual's specific genetic background and pathologic characteristics. The application of precision medicine is expected to improve treatment outcomes, reduce unwanted side effects, and optimize resource allocation. Meanwhile, technological advances, particularly artificial intelligence, machine learning, and virtual reality, are expected to revolutionize the way ASDs are diagnosed, monitored, and treated. These technologies are capable of delivering customized learning and treatment programs that enhance the acceptability and effectiveness of interventions. In addition, interdisciplinary research will be strengthened, and social policies and public health strategies will focus more on early screening and intervention, as well as increasing public awareness and understanding of ASD. Most importantly, the future of ASD prevention and treatment will place greater emphasis on the needs of patients and families, promote social integration and employment of patients, and improve their quality of life. As society's awareness of diversity and inclusion increases, individuals with ASD will receive more support and respect and enjoy fuller opportunities for social participation.
Author information
Authors and affiliations.
Department of Rehabilitation, The Second Affiliated Hospital of Shandong First Medical University, Taian, Shandong, China
Lei Qin, Wenjing Ning & Mengmeng Cui
Department of Intensive Care Medicine, Feicheng People’s Hospital, Taian, Shandong, China
Haijiao Wang
Department of Central Laboratory, The Affiliated Taian City Central Hospital of Qingdao University, Taian, China
You can also search for this author in PubMed Google Scholar
Contributions
LQ, HW and WN wrote the draft of the manuscript. MC and QW revised this manuscript. All the listed authors have made a substantial, direct, and intellectual contribution to the work, and approved its publication.
Corresponding authors
Correspondence to Mengmeng Cui or Qian Wang .
Ethics declarations
Ethical approval and consent to participate.
Not applicable.
Competing interests
The authors declare there are no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Qin, L., Wang, H., Ning, W. et al. New advances in the diagnosis and treatment of autism spectrum disorders. Eur J Med Res 29 , 322 (2024). https://doi.org/10.1186/s40001-024-01916-2
Download citation
Received : 17 April 2024
Accepted : 01 June 2024
Published : 10 June 2024
DOI : https://doi.org/10.1186/s40001-024-01916-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Autism spectrum disorder (ASD)
- Diagnostic methods
- Treatment strategies
- Precision medicine
- Emerging biotechnology
European Journal of Medical Research
ISSN: 2047-783X
- General enquiries: [email protected]
- Download PDF
- CME & MOC
- Share X Facebook Email LinkedIn
- Permissions
Autism Spectrum Disorder : A Review
- 1 Department of Psychiatry and Behavioral Sciences, UCSF Weill Institute for Neurosciences, University of California, San Francisco
- Special Communication Screening for Autism Spectrum Disorder in Young Children Albert L. Siu, MD, MSPH; and the US Preventive Services Task Force (USPSTF); Kirsten Bibbins-Domingo, PhD, MD, MAS; David C. Grossman, MD, MPH; Linda Ciofu Baumann, PhD, RN, APRN; Karina W. Davidson, PhD, MASc; Mark Ebell, MD, MS; Francisco A. R. García, MD, MPH; Matthew Gillman, MD, SM; Jessica Herzstein, MD, MPH; Alex R. Kemper, MD, MPH, MS; Alex H. Krist, MD, MPH; Ann E. Kurth, PhD, RN, MSN, MPH; Douglas K. Owens, MD, MS; William R. Phillips, MD, MPH; Maureen G. Phipps, MD, MPH; Michael P. Pignone, MD, MPH JAMA
- JAMA Patient Page Screening for Autism Spectrum Disorder Jill Jin, MD, MPH JAMA
- JAMA Patient Page Patient Information: Autism Spectrum Disorder Rebecca Voelker, MSJ JAMA
- Review The Emerging Clinical Neuroscience of Autism Spectrum Disorder Rebecca A. Muhle, MD, PhD; Hannah E. Reed, MD; Katharine A. Stratigos, MD; Jeremy Veenstra-VanderWeele, MD JAMA Psychiatry
- Original Investigation Association of Allergies With Autism Spectrum Disorder Guifeng Xu, MD; Linda G. Snetselaar, PhD; Jin Jing, MD, PhD; Buyun Liu, MD, PhD; Lane Strathearn, MBBS, FRACP, PhD; Wei Bao, MD, PhD JAMA Network Open
- Research Letter Racial and Ethnic Differences in Rates and Age of Diagnosis of Autism Spectrum Disorder Hoangmai H. Pham, MD, MPH; Neil Sandberg, MS; Jeff Trinkl, MD; Johnston Thayer, MBA, RN JAMA Network Open
- Original Investigation Concordance of Diagnosis of ASD Made by Pediatricians vs a Multidisciplinary Specialist Team Melanie Penner, MSc, MD; Lili Senman, MA; Lana Andoni, MSc; Annie Dupuis, PhD; Evdokia Anagnostou, MD; Shawn Kao, MD; Abbie Solish, PhD; Michelle Shouldice, MEd, MD; Genevieve Ferguson, MEd; Jessica Brian, PhD JAMA Network Open
- Original Investigation Association Between Autism Spectrum Disorders and Cardiometabolic Diseases Chathurika S. Dhanasekara, MD, MS, PhD; Dominic Ancona, M-PAS; Leticia Cortes, M-PAS; Amy Hu, M-PAS; Afrina H. Rimu, MD, MS; Christina Robohm-Leavitt, M-PAS, DMSc; Drew Payne, DO; Sarah M. Wakefield, MD; Ann M. Mastergeorge, PhD; Chanaka N. Kahathuduwa, MD, MPhil, PhD JAMA Pediatrics
Importance Autism spectrum disorder (ASD), characterized by deficits in social communication and the presence of restricted, repetitive behaviors or interests, is a neurodevelopmental disorder affecting approximately 2.3% children aged 8 years in the US and approximately 2.2% of adults. This review summarizes evidence on the diagnosis and treatment of ASD.
Observations The estimated prevalence of ASD has been increasing in the US, from 1.1% in 2008 to 2.3% in 2018, which is likely associated with changes in diagnostic criteria, improved performance of screening and diagnostic tools, and increased public awareness. No biomarkers specific to the diagnosis of ASD have been identified. Common early signs and symptoms of ASD in a child’s first 2 years of life include no response to name when called, no or limited use of gestures in communication, and lack of imaginative play. The criterion standard for the diagnosis of ASD is a comprehensive evaluation with a multidisciplinary team of clinicians and is based on semistructured direct observation of the child’s behavior and semistructured caregiver interview focused on the individual’s development and behaviors using standardized measures, such as the Autism Diagnostic Observation Schedule-Second Edition and the Autism Diagnostic Interview. These diagnostic measures have sensitivity of 91% and 80% and specificity of 76% and 72%, respectively. Compared with people without ASD, individuals with ASD have higher rates of depression (20% vs 7%), anxiety (11% vs 5%), sleep difficulties (13% vs 5%), and epilepsy (21% with co-occurring intellectual disability vs 0.8%). Intensive behavioral interventions, such as the Early Start Denver Model, are beneficial in children 5 years or younger for improvement in language, play, and social communication (small to medium effect size based on standardized mean difference). Pharmacotherapy is indicated for co-occurring psychiatric conditions, such as emotion dysregulation or attention-deficit/hyperactivity disorder. Risperidone and aripiprazole can improve irritability and aggression (standardized mean difference of 1.1, consistent with a large effect size) compared with placebo. Psychostimulants are effective for attention-deficit/hyperactivity disorder (standardized mean difference of 0.6, consistent with a moderate effect size) compared with placebo. These medications are associated with adverse effects including, most commonly, changes in appetite, weight, and sleep.
Conclusions and Relevance ASD affects approximately 2.3% of children aged 8 years and approximately 2.2% of adults in the US. First-line therapy consists of behavioral interventions, while co-occurring psychiatric conditions, such as anxiety or aggression, may be treated with specific behavioral therapy or medication.
Read More About
Hirota T , King BH. Autism Spectrum Disorder : A Review . JAMA. 2023;329(2):157–168. doi:10.1001/jama.2022.23661
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Neurodevelopmental Disorders
- Developmental Pediatrics
- Developmental Disorders
- Autism Spectrum Disorders
Autism Spectrum Disorder: Review Article
- Medico-Legal Update 20(2):324-329
- 20(2):324-329

- Al Muthanna University

Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations
- Norwahidah Mohamad Yazid

- Zainura Binti Idrus

- Doaa Elsayed
- Sergio E. Starkstein

- AM J MED GENET B

- Richard M. Kubina

- J NEUROINFLAMM

- Abraham Reichenberg

- Mark F O'Reilly

- Myriam Suárez

- Judy S. Reilly
- Patricia A Stewart
- Susan L. Hyman
- Brianne Schmidt

- John Bankart
- Howard Meltzer
- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
This week: the arXiv Accessibility Forum
Help | Advanced Search
Computer Science > Machine Learning
Title: mcdgln: masked connection-based dynamic graph learning network for autism spectrum disorder.
Abstract: Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder characterized by complex physiological processes. Previous research has predominantly focused on static cerebral interactions, often neglecting the brain's dynamic nature and the challenges posed by network noise. To address these gaps, we introduce the Masked Connection-based Dynamic Graph Learning Network (MCDGLN). Our approach first segments BOLD signals using sliding temporal windows to capture dynamic brain characteristics. We then employ a specialized weighted edge aggregation (WEA) module, which uses the cross convolution with channel-wise element-wise convolutional kernel, to integrate dynamic functional connectivity and to isolating task-relevant connections. This is followed by topological feature extraction via a hierarchical graph convolutional network (HGCN), with key attributes highlighted by a self-attention module. Crucially, we refine static functional connections using a customized task-specific mask, reducing noise and pruning irrelevant links. The attention-based connection encoder (ACE) then enhances critical connections and compresses static features. The combined features are subsequently used for classification. Applied to the Autism Brain Imaging Data Exchange I (ABIDE I) dataset, our framework achieves a 73.3\% classification accuracy between ASD and Typical Control (TC) groups among 1,035 subjects. The pivotal roles of WEA and ACE in refining connectivity and enhancing classification accuracy underscore their importance in capturing ASD-specific features, offering new insights into the disorder.
| Comments: | 8 pages, 7 figures |
| Subjects: | Machine Learning (cs.LG); Artificial Intelligence (cs.AI) |
| Cite as: | [cs.LG] |
| (or [cs.LG] for this version) | |
| Focus to learn more arXiv-issued DOI via DataCite |
Submission history
Access paper:.
- HTML (experimental)
- Other Formats
References & Citations
- Google Scholar
- Semantic Scholar
BibTeX formatted citation
Bibliographic and Citation Tools
Code, data and media associated with this article, recommenders and search tools.
- Institution
arXivLabs: experimental projects with community collaborators
arXivLabs is a framework that allows collaborators to develop and share new arXiv features directly on our website.
Both individuals and organizations that work with arXivLabs have embraced and accepted our values of openness, community, excellence, and user data privacy. arXiv is committed to these values and only works with partners that adhere to them.
Have an idea for a project that will add value for arXiv's community? Learn more about arXivLabs .
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
September 12, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
How parents and caregivers can evaluate the research on MERT and other potential autism treatments
by Corinne Purtill, Los Angeles Times

As diagnoses of autism spectrum disorder have increased in the last two decades, so have the number of experimental and off-label therapies seeking to address the condition.
For parents navigating the complex and often contradictory landscape of autism interventions—while also balancing medical appointments, educational specialists and countless other family needs—evaluating these treatments can be daunting.
Experts in autism research talked to The Times about what parents and patients should watch for when evaluating a potential new treatment—for autism or any other condition.
Take testimonials with a grain of salt
Firsthand accounts of a therapy's life-changing effects can be powerfully compelling. But such stories alone can't indicate how effective a treatment will be for anyone else, autism experts said.
"Be wary of therapies that are sold to you with testimonials. If you go to a clinic website and they have dozens of quotes from parents saying 'This changed my child's life in XYZ ways,' that isn't the same as evidence," said Zoe Gross of the Autistic Self Advocacy Network, a nonprofit group run by and for autistic adults.
"If the main way something's advertised is through testimonials, it may be because there isn't research, or what research was done showed it wasn't effective."
Without accompanying data, there is no way to know whether any patient's experience with a treatment is typical or an outlier. A therapy could have only a 1% success rate, Gross said, and still yield dozens of positive outcomes once thousands of people have tried it.
Former patient stories can be a starting point for an exploration of whether a therapy is right for someone, doctors said, but the exploration shouldn't end there.
"There's an old saying in medicine," said Dr. Andrew Leuchter, director of UCLA's TMS Clinical and Research Service. "The plural of anecdote is not data."
Look for—and at—the research
"Right now, it's really sexy to call yourself 'evidence-based,'" said Dr. David Celiberti, executive director of the nonprofit Assn. for Science in Autism Treatment. "For a consumer, that's amazing. You hear 'evidence-based' and of course, you're going to be drawn to it. But people are using that term very loosely."
In the case of magnetic e-resonance therapy, or MERT, its developer Wave Neuroscience features on its website a library of research. Similar links feature on the sites of many licensee clinics.
Most of the publications related to autism cited by MERT clinics—and, at times, by Wave—are either limited in scope or only tangentially related to the therapy, a half-dozen experts said, including some whose work is cited.
One of them, for example, is a brief 2016 article from the Austin Journal of Autism and Related Disabilities titled "The Potential of Magnetic Resonant Therapy in Children with Autism Spectrum Disorder."
Its authors and advisors said they were surprised to learn the paper was being used to advertise the treatment. The paper contains no data or original research and concludes only that MERT could be studied further as an autism therapy without risk of serious harm.
"This isn't an evidence-based paper. It's an opinion piece about the possibilities of this technology," said Dr. John Crawford, a neurologist at Children's Hospital of Orange County and a co-author of the paper. "It's not that impactful from a scientific perspective."
Who else has verified these findings?
Many MERT clinics feature a 2014 electronic poster presentation that examines data from the charts of 141 children who received transcranial magnetic stimulation , the therapy on which MERT is based, for autism.
Until March, Wave featured the poster on its website and highlighted that 59.1% of 44 participants who completed 12 months of treatment improved their scores on the Childhood Autism Rating Scale, an assessment tool used to gauge symptom severity.
A closer look at the report shows that after five days of treatment, 38 patients were dropped from the analysis because their symptoms either showed no improvement or worsened. One had a seizure during treatment.
The authors excluded dozens more patients for various reasons. Of the remaining 44 patients, 26 saw improvement while getting the treatment. That was 59.1% of those remaining, as the poster said, but only 18.4% of the total study population.
The write-up also notes that many of those 26 children were receiving other therapies at the same time that may have been responsible for some or all of the improvements.
Posters are typically prepared as a way to highlight findings at professional conferences and "cannot be interpreted as having undergone rigorous peer review," said USC neurosurgeon Dr. Charles Liu, a co-author on the poster who is not affiliated with Wave or any MERT clinics.
"The main point of the abstract is and remains that more rigorous studies must [be] done."
If research shows changes, how do you know the therapy caused it?
Wave and licensees also highlight a 2022 paper by a technician at a licensee clinic in Australia who is also a doctoral candidate at Australia's University of the Sunshine Coast.
It looks at data from 28 patients at two MERT clinics in Australia whose brains showed "significant improvement" in their individual alpha frequency waves after treatment.
Although some previous research has found correlations between atypical alpha wave frequency and autism diagnoses, six scientists told The Times that there isn't yet enough evidence to understand how changes in alpha waves affect autistic traits, or any scientific consensus on whether "improvement" in this pattern of brain activity has any meaningful effect on autistic behaviors.
The report is a retrospective chart review, which examines existing data from patients' medical records and is often used to identify interesting outcomes worthy of further study.
By design, it does not include a control group, which is what allows researchers to identify whether any changes they see are related to the variable they are studying. Its authors noted in the paper that findings are preliminary and require further study.
"Because this was not a controlled trial or study, [the cause of the changes] could have been anything including placebo effect, any additional therapies the children were receiving, etc.," said Lindsay Oberman, director of the Neurostimulation Research Program at the National Institute of Mental Health.
Medical research follows a hierarchy of evidence. At the bottom are anecdotes and observations: valid points of information that alone aren't enough to draw broad conclusions from.
Above that are observational studies that collect and analyze preexisting data in a systematic way. And at the top are randomized controlled trials, which are designed to eliminate as much bias as possible from the experiment and ensure that the thing being studied is responsible for any changes observed.
"Families need to know that there is this gold standard for studies—to make sure that something works to help people with autism, it needs to have what's called a randomized controlled trial ," said Alycia Halladay, chief science officer at the Autism Science Foundation.
2024 Los Angeles Times. Distributed by Tribune Content Agency, LLC.
Explore further
Feedback to editors

Rapid blood diagnostic test developed for amyotrophic lateral sclerosis

Automated insulin delivery technology helps marathon runners with type 1 diabetes
3 hours ago

Next-gen gene therapy vector for muscle diseases uses AI predictive methodology to improve efficacy and safety

Gut reaction: Low levels of manganese can aggravate inflammatory bowel disease
4 hours ago

A novel neural explanation for choking under pressure

Novel technology enabling sampling of liquids in confined spaces could aid early detection of cancer

Mouse study finds sex-based differences in how brains handle threats

A new biomarker makes it easier to distinguish between Alzheimer's and primary tauopathy
5 hours ago

Supported youth become supportive adults, researchers find
6 hours ago

Exposure to air pollution during pregnancy increases postpartum depression risk for at least three years, study finds
7 hours ago
Related Stories


Can robot-inspired computer-assisted therapy benefit children with autism?
May 15, 2024

Motor skills, sensory features differ in autism with, without ADHD
Mar 22, 2024

Adults with ADHD exhibit camouflaging behavior
Feb 21, 2024

Survey heightens concern about pandemic's impact on education of students with autism
Feb 17, 2022

Research shows in-school occupational therapy creates positive education experiences for kids with autism
Aug 16, 2023

Undiagnosed autistic traits common among youths with substance use disorders
Jan 24, 2022
Recommended for you

Study debunks theory linking autism to changes in brain's amygdala
Sep 4, 2024

Eating fish, not omega-3 supplements during pregnancy associated with lower likelihood of autism diagnosis
Sep 3, 2024

Collaborative research cracks the autism code, making the neurodivergent brain visible
Aug 28, 2024

Autistic traits, behavioral problems in 7-year-olds linked with gender nonconforming play

Study shows Dungeons and Dragons can help autistic people gain confidence and find their inner hero
Aug 27, 2024

Autism spectrum disorders linked to neurotransmitter switching in the brain
Aug 22, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Exp Neurobiol
- v.25(1); 2016 Feb
A Short Review on the Current Understanding of Autism Spectrum Disorders
Hye ran park.
1 Department of Neurosurgery, Seoul National University Hospital, Seoul 03080, Korea.
Jae Meen Lee
Hyo eun moon, dong soo lee.
2 Department of Nuclear Medicine, Seoul National University College of Medicine, Seoul 03080, Korea.
Bung-Nyun Kim
3 Division of Child and Adolescent Psychiatry, Department of Psychiatry, Seoul National University College of Medicine, Seoul 03080, Korea.
Jinhyun Kim
4 Center for Functional Connectomics, Korea Institute of Science and Technology (KIST), Seoul 02792, Korea.
Dong Gyu Kim
Sun ha paek.
Autism spectrum disorder (ASD) is a set of neurodevelopmental disorders characterized by a deficit in social behaviors and nonverbal interactions such as reduced eye contact, facial expression, and body gestures in the first 3 years of life. It is not a single disorder, and it is broadly considered to be a multi-factorial disorder resulting from genetic and non-genetic risk factors and their interaction. Genetic studies of ASD have identified mutations that interfere with typical neurodevelopment in utero through childhood. These complexes of genes have been involved in synaptogenesis and axon motility. Recent developments in neuroimaging studies have provided many important insights into the pathological changes that occur in the brain of patients with ASD in vivo. Especially, the role of amygdala, a major component of the limbic system and the affective loop of the cortico-striatothalamo-cortical circuit, in cognition and ASD has been proved in numerous neuropathological and neuroimaging studies. Besides the amygdala, the nucleus accumbens is also considered as the key structure which is related with the social reward response in ASD. Although educational and behavioral treatments have been the mainstay of the management of ASD, pharmacological and interventional treatments have also shown some benefit in subjects with ASD. Also, there have been reports about few patients who experienced improvement after deep brain stimulation, one of the interventional treatments. The key architecture of ASD development which could be a target for treatment is still an uncharted territory. Further work is needed to broaden the horizons on the understanding of ASD.
INTRODUCTION
Autism spectrum disorder (ASD) is a set of neurodevelopmental disorders characterized by a lack of social interaction, verbal and nonverbal communication in the first 3 years of life. The distinctive social behaviors include an avoidance of eye contact, problems with emotional control or understanding the emotions of others, and a markedly restricted range of activities and interests [ 1 ]. The current prevalence of ASD in the latest large-scale surveys is about 1%~2% [ 2 , 3 ]. The prevalence of ASD has increased in the past two decades [ 4 ]. Although the increase in prevalence is partially the result of changes in DSM diagnostic criteria and younger age of diagnosis, an increase in risk factors cannot be ruled out [ 5 , 6 ]. Studies have shown a male predominance; ASD affects 2~3 times more males than females [ 2 , 3 , 7 ]. This diagnostic bias towards males might result from under-recognition of females with ASD [ 8 ]. Also, some researchers have suggested the possibility that the female-specific protective effects against ASD might exist [ 9 ].
A Swiss psychiatrist, Paul Eugen Bleuler used the term "autism" to define the symptoms of schizophrenia for the first time in 1912 [ 10 ]. He derived it from the Greek word αὐτὀς (autos), which means self. Hans Asperger adopted Bleuler's terminology "autistic" in its modern sense to describe child psychology in 1938. Afterwards, he reported about four boys who did not mix with their peer group and did not understand the meaning of the terms 'respect' and 'polite', and regard for the authority of an adult. The boys also showed specific unnatural stereotypic movement and habits. Asperger describe this pattern of behaviors as "autistic psychopathy", which is now called as Asperger's Syndrome [ 11 ]. The person who first used autism in its modern sense is Leo Kanner. In 1943, he reported about 8 boys and 3 girls who had "an innate inability to form the usual, biologically provided affective contact with people", and introduced the label early infantile autism [ 12 ]. Hans Asperger and Leo Kanner have been considered as those who designed the basis of the modern study of autism.
Most recently, the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) adopted the term ASD with a dyadic definition of core symptoms: early-onset of difficulties in social interaction and communication, and repetitive, restricted behaviors, interests, or activities [ 13 ]. Atypical language development, which had been included into the triad of ASD, is now regarded as a co-occurring condition.
As stated earlier, the development of the brain in individuals with ASD is complex and is mediated by many genetic and environmental factors, and their interactions. Genetic studies of ASD have identified mutations that interfere with typical neurodevelopment in utero through childhood. These complexes of genes have been involved in synaptogenesis and axon motility. Also, the resultant microstructural, macrostructural, and functional abnormalities that emerge during brain development create a pattern of dysfunctional neural networks involved in socioemotional processing. Microstructurally, an altered ratio of short- to long-diameter axons and disorganization of cortical layers are observed. Macrostructurally, MRI studies assessing brain volume in individuals with ASD have consistently shown cortical and subcortical gray matter overgrowth in early brain development. Functionally, resting-state fMRI studies show a narrative of widespread global underconnectivity in socioemotional networks, and task-based fMRI studies show decreased activation of networks involved in socioemotional processing. Moreover, electrophysiological studies demonstrate alterations in both resting-state and stimulus-induced oscillatory activities in patients with ASD [ 14 ].
The well-conserved sets of genes and genetic pathways were implicated in ASD, many of which contribute toward the formation, stabilization, and maintenance of functional synapses. Therefore, these genetic aspects coupled with an in-depth phenotypic analysis of the cellular and behavioral characteristics are essential to unraveling the pathogenesis of ASD. The number of genes already discovered in ASD holds the promise to translate the knowledge into designing new therapeutic interventions. Also, the fundamental research using animal models is providing key insights into the various facets of human ASD. However, a better understanding of the genetic, molecular, and circuit level aberrations in ASD is still needed [ 15 ].
Neuroimaging studies have provided many important insights into the pathological changes that occur in the brain of patients with ASD in vivo. Importantly, ASD is accompanied by an atypical path of brain maturation, which gives rise to differences in neuroanatomy, functioning, and connectivity. Although considerable progress has been made in the development of animal models and cellular assays, neuroimaging approaches allow us to directly examine the brain in vivo, and to probably facilitate the development of a more personalized approach to the treatment of ASD [ 16 ].
ASD is not a single disorder. It is now broadly considered to be a multi-factorial disorder resulting from genetic and non-genetic risk factors and their interaction.
Genetic causes including gene defects and chromosomal anomalies have been found in 10%~20% of individuals with ASD [ 17 , 18 ]. Siblings born in families with an ASD subject have a 50 times greater risk of ASD, with a recurrence rate of 5%~8% [ 19 ]. The concordance rate reaches up to 82%~92% in monozygotic twins, compared with 1%~10% in dizygotic twins. Genetic studies suggested that single gene mutations alter developmental pathways of neuronal and axonal structures involved in synaptogenesis [ 20 , 21 , 22 ]. In the cases of related with fragile X syndrome and tuberous sclerosis, hyperexcitability of neocortical circuits caused by alterations in the neocortical excitatory/inhibitory balance and abnormal neural synchronization is thought to be the most probable mechanisms [ 23 , 24 ]. Genome-wide linkage studies suggested linkages on chromosomes 2q, 7q, 15q, and 16p as the location of susceptibility genes, although it has not been fully elucidated [ 25 , 26 ]. These chromosomal abnormalities have been implicated in the disruption of neural connections, brain growth, and synaptic/dendritic morphology [ 27 , 28 , 29 ]. Metabolic errors including phenylketonuria, creatine deficiency syndromes, adenylosuccinate lyase deficiency, and metabolic purine disorders are also account for less than 5% of individuals with ASD [ 30 ]. Recently, the correlation between cerebellar developmental patterning gene ENGRAILED 2 and autism was reported [ 31 ]. It is the first genetic allele that contributes to ASD susceptibility in as many as 40% of ASD cases. Other genes such as UBE3A locus, GABA system genes, and serotonin transporter genes have also been considered as the genetic factors for ASD [ 18 ].
Diverse environmental causative elements including pre-natal, peri-natal, and post-natal factors also contribute to ASD [ 32 ]. Prenatal factors related with ASD include exposure to teratogens such as thalidomide, certain viral infections (congenital rubella syndrome), and maternal anticonvulsants such as valproic acid [ 33 , 34 ]. Low birth weight, abnormally short gestation length, and birth asphyxia are the peri-natal factors [ 34 ]. Reported post-natal factors associated with ASD include autoimmune disease, viral infection, hypoxia, mercury toxicity, and others [ 33 , 35 , 36 ]. Table 1 summarizes the known and putative ASD-related genes and environmental factors contributing to the ASD.

In recent years, some researchers suggest that ASD is the result of complex interactions between genetic and environmental risk factors [ 37 ]. Understanding the interaction between genetic and environmental factors in the pathogenesis of ASD will lead to optimal treatment strategy.
Clinical features and Diagnosis
ASD is typically noticed in the first 3 years of life, with deficits in social behaviors and nonverbal interactions such as reduced eye contact, facial expression, and body gestures [ 1 ]. Children also manifest with non-specific symptoms such as unusual sensory perception skills and experiences, motor clumsiness, and insomnia. Associated phenomena include mental retardation, emotional indifference, hyperactivity, aggression, self-injury, and repetitive behaviors such as body rocking or hand flapping. Repetitive, stereotyped behaviors are often accompanied by cognitive impairment, seizures or epilepsy, gastrointestinal complaints, disturbedd sleep, and other problems. Differential diagnosis includes childhood schizophrenia, learning disability, and deafness [ 38 , 39 ].
ASD is diagnosed clinically based on the presence of core symptoms. However, caution is required when diagnosing ASD because of non-specific manifestations in different age groups and individual abilities in intelligence and verbal domains. The earliest nonspecific signs recognized in infancy or toddlers include irritability, passivity, and difficulties with sleeping and eating, followed by delays in language and social engagement. In the first year of age, infants later diagnosed with ASD cannot be easily distinguished from control infants. However, some authors report that about 50% of infants show behavioral abnormalities including extremes of temperament, poor eye contact, and lack of response to parental voices or interaction. At 12 months of age, individuals with ASD show atypical behaviors, across the domains of visual attention, imitation, social responses, motor control, and reactivity [ 40 ]. There is also report about atypical language trajectories, with mild delays at 12 months progressing to more severe delays by 24 months [ 40 ]. By 3 years of age, the typical core symptoms such as lack of social communication and restricted/repetitive behaviors and interests are manifested. ASD can be easily differentiated from other psychosocial disorders in late preschool and early school years.
Amygdala and ASD
The frontal and temporal lobes are the markedly affected brain areas in the individuals with ASD. In particular, the role of amygdala in cognition and ASD has been proved in numerous neuropathological and neuroimaging studies. The amygdala located the medial temporal lobe anterior to the hippocampal formation has been thought to have a strong association with social and aggressive behaviors in patients with ASD [ 41 , 42 ]. The amygdala is a major component of the limbic system and affective loop of the cortico-striato-thalamo-cortical circuit [ 43 ].
The amygdala has 2 specific functions including eye gaze and face processing [ 44 ]. The lesion of the amygdala results in fear-processing, modulation of memory with emotional content, and eye gaze when looking at human face [ 45 , 46 , 47 ]. The findings in individuals with amygdala lesion are similar to the phenomena in ASD. The amygdala receives highly processed somatosensory, visual, auditory, and all types of visceral inputs. It sends efferents through two major pathways, the stria terminalis and the ventral amygdalofugal pathway.
The amygdala comprises a collection of 13 nuclei. Based on histochemical analyses, these 13 nuclei are divided into three primary subgroups: the basolateral (BL), centromedial (CM), and superficial groups [ 42 ]. The BL group attributes amygdala to have a role as a node connecting sensory stimuli to higher social cognition level. It links the CM and superficial groups, and it has reciprocal connection with the orbitofrontal cortex, anterior cingulate cortex (ACC), and the medial prefrontal cortex (mPFC) [ 48 ]. The BL group contains neurons responsive to faces and actions of others, which is not found in the other two groups of amygdala [ 49 , 50 ]. The CM group consists of the central, medial, cortical nuclei, and the periamygdaloid complex. It innervates many of the visceral and autonomic effector regions of the brain stem, and provides a major output to the hypothalamus, thalamus, ventral tegmental area, and reticular formation [ 51 ]. The superficial group includes the nucleus of the lateral olfactory tract [ 42 ].
Neurochemistrial studies revealed high density of benzodiazepine/GABAa receptors and a substantial set of opiate receptors in the amygdala. It also includes serotonergic, dopaminergic, cholinergic, and noradrenergic cell bodies and pathways [ 52 ]. Since some patients with temporal epilepsy and aggressive behavior experienced improvement in aggressiveness after bilateral stereotactic ablation of basal and corticomedial amygdaloid nuclei, the role of amygdala in emotional processing, especially rage processing has been investigated [ 53 , 54 , 55 , 56 ]. Some evidences for the amygdala deficit in patients with ASD have been suggested. Post-mortem studies found the pathology in the amygdala of individuals with ASD compared to age- and sex-matched controls [ 57 , 58 , 59 ]. Small neuronal size and increased cell density in the cortical, medial, and central nuclei of the amygdala were detected in ASD patients.
Several studies proposed the use of an animal model to confirm the evidence for the association between amygdala and ASD [ 60 , 61 ]. Despite the limitation which stems from the need to prove higher order cognitive disorder, the studies suggested that disease-associated alterations in the temporal lobes during experimental manipulations of the amygdala in animals have produced some symptoms of ASD [ 62 ]. Especially, the Kluver-Bucy syndrome, which is caused by bilateral damage to the anterior temporal lobes in monkeys, has characteristic manifestations similar to ASD [ 63 , 64 ]. Monkeys with the Kluver-Bucy syndrome shows absence of social chattering, lack of facial expression, absence of emotional reactions, repetitive abnormal movement patterns, and increased aggression. Sajdyk et al. performed experiments on rats and discovered that physiological activation of the BL nucleus of the amygdala by blocking tonic GABAergic inhibition or enhancing glutamate or the stress-associated peptide corticotropin-releasing factor (CRF)-mediated excitation caused reduction in social behaviors [ 65 ]. On the contrary, lesioning of the amygdala or blocking amygdala excitability with glutamate antagonist increased dyadic social interactions [ 60 ]. Besides animals, humans who underwent lesioning of the amygdala showed impairments in social judgment. This phenomenon is called acquired ASD [ 66 , 67 , 68 ]. The pattern of social deficits was similar in idiopathic and acquired ASD [ 69 ]. Felix-Ortiz and Tye sought to understand the role of projections from the BL amygdala to the ventral hippocampus in relation to behavior. Their study using mice showed that the BLS-ventral hippocampus pathway involved in anxiety plays a role in the mediation of social behavior as well [ 70 ].
The individuals with temporal lobe tumors involving the amygdala and hippocampus provide another evidence of the correlation between the amygdala and ASD. Some authors reported that patients experienced autistic symptoms after temporal lobe was damaged by a tumor [ 71 , 72 ]. Also, individuals with tuberous sclerosis experienced similar symptoms including facial expression due to a temporal lobe hamartoma [ 73 ].
Although other researchers failed to find structural abnormalities in the mesial temporal lobe of autistic subjects by performing magnetic resonance imaging (MRI) studies [ 74 , 75 , 76 ], recent development in neuroimaging has facilitated the investigation of amygdala pathology in ASD. Studies using structural MRI estimated volumes of the amygdala and related structures in individuals with ASD and age-, gender, and verbal IQ-matched healthy controls [ 77 ]. Increase in bilateral amygdala volume and reduction in hippocampal and parahippocampal gyrus volumes were noted in individuals with ASD. Also, the lateral ventricles and intracranial volumes were significantly increased in the autistic subjects; however, overall temporal lobe volumes were similar between the ASD and control groups.
There was a significant difference in the whole brain voxel-based scans of individuals with ASD and control groups [ 78 ]. Individuals with ASD showed decreased gray matter volume in the right paracingulate sulcus, the left occipito-temporal cortex, and the left inferior frontal sulcus. On the contrary, the gray matter volume in the bilateral cerebellum was increased. Otherwise, they showed increased volume in the left amygdala/periamygdaloid cortex, the right inferior temporal gyrus, and the middle temporal gyrus.
Recently, the development of functional neuroimaging also provided some evidence for the correlation between amygdala deficit and ASD. A study using Technetium-99m (Tc-99m) single-photon emission computed tomography (SPECT) found that regional cerebral blood flow (rCBF) was decreased in the bilateral insula, superior temporal gyri, and left prefrontal cortices in individuals with ASD compared to age- and gender-matched controls with mental retardation [ 79 ]. Also, the authors found that rCBF in both the right hippocampus and amygdala was correlated with a behavioral rating subscale.
On proton magnetic resonance spectroscopy (MRS) in the right hippocampal-amygdala region and the left cerebellar hemisphere, autistic subjects showed decreased level of N-acetyl aspartate (NAA) in both areas [ 80 ]. There was no difference in the level of the other metabolites, such as creatine and choline. This study implies that a decreased level of NAA might be associated with neuronal hypofunction or immature neurons.
These findings support the claim that amygdala might be a key structure in the development of ASD and a target for the management of the disease.
Prefrontal cortex and ASD
Frontal lobe has been considered as playing an important role in higher-level control and a key structure associated with autism. Individuals with frontal lobe deficit demonstrate higher-order cognitive, language, social, and emotion dysfunction, which is deficient in autism [ 81 ]. Recently, neuroimaging and neuropsychological studies have attempted to delineate distinct regions of prefrontal cortex supporting different aspects of executive function. Some authors have reported that the excessive rates of brain growth in infants with ASD, which is mainly contributed by the increase of frontal cortex volume [ 82 , 83 ]. Especially, the PFC including Brodmann areas 8, 9, 10, 11, 44, 45, 46, and 47 has been noted for the structure related with ASD [ 84 ]. The PFC is cytoarchitectonically defined as the presence of a cortical granular layer IV [ 85 ], and anatomically refers to the regions of the cerebral cortex that are anterior to premotor cortex and the supplementary motor area [ 86 ]. The PFC has extensive connections with other cortical, subcortical and brain stem sites [ 87 ]. It receives inputs from the brainstem arousal systems, and its function is particularly dependent on its neurochemical environment [ 88 ].
The PFC is broadly divided into the medial PFC (mPFC) and the lateral PFC (lPFC). The mPFC is further divided into four distinct regions: medial precentral cortex, anterior cingulate cortex, prelimbic and infralimbic prefrontal cortex [ 89 ]. While the lPFC is thought to support cognitive control process [ 90 ], the mPFC has reciprocal connections with brain regions involved in emotional processing (amygdala), memory (hippocampus) and higher-order sensory regions (within temporal cortex) [ 91 ]. This involvement of mPFC in social cognition and interaction implies that mPFC might be a key region in understanding self and others [ 92 ].
The mPFC involves in fear learning and extinction by reciprocal synaptic connections with the basolateral amygdala [ 93 , 94 ]. It is believed that the mPFC regulates and controls amygdala output and the accompanying behavioral phenomena [ 95 , 96 ]. Previous authors investigated how memory processing is regulated by interactions between BLA and mPFC by means of functional disconnection [ 97 , 98 ]. Disturbed communication within amygdala-mPFC circuitry caused deficits in memory processing. These informations provide support for a role of the mPFC in the development of ASD.
Nucleus Accumbens and ASD
Besides amygdala, nucleus accumbens (NAc) is also considered as the key structure which is related with the social reward response in ASD. NAc borders ventrally on the anterior limb of the internal capsule, and the lateral subventricular fundus of the NAc is permeated in rostral sections by internal capsule fiber bundles. The rationale for NAc to be considered as the potential target of DBS for ASD is its predominant role in modulating the processing of reward and pleasure [ 99 ]. Anticipation of rewarding stimuli recruits the NAc as well as other limbic structures, and the experience of pleasure activates the NAc as well as the caudate, putamen, amygdala, and VMPFC [ 100 , 101 , 102 ]. It is well known that dysfunction of NAc regarding rewarding stimuli in subjects with depression. Bewernick et al. demonstrated antidepressant effects of NAc-DBS in 5 of the 10 patients suffering from severe treatment-resistant depression [ 103 ].
Two groups reported about the neural basis of social reward processing in ASD. Schmitz et al. examined responses to a task that involved monetary reward. They investigated the neural substrates of reward feedback in the context of a sustained attention task, and found increased activation in the left anterior cingulate gyrus and left mid-frontal gyrus on rewarded trials in ASD [ 104 ]. Scott-Van Zeeland et al. investigated the neural correlates of rewarded implicit learning in children with ASD using both social and monetary rewards. They found diminished ventral striatal response during social, but not monetary, rewarded learning [ 105 ]. According to them, activity within the ventral striatum predicted social reciprocity within the control group, but not within the ASD group.
Anticipation of pleasurable stimuli recruits the NAc, whereas the experience of pleasure activates VMPFC [ 106 ]. NAc is activated by incentive motivation to reach salient goals [ 106 ]. Increased activation in the left anterior cingulate gyrus and left mid-frontal gyrus was noted during both the anticipatory and consummatory phase of the reward response [ 104 , 107 , 108 ]. However, the activity within the ventral striatum was decreased in autistic subjects, which caused impairment in social reciprocity [ 105 ].
These findings indicate that reward network function in ASD is contingent on both the temporal phase of the response and the type of reward processed, suggesting that it is critical to assess the temporal chronometry of responses in a study of reward processing in ASD. NAc might be one of the candidates as a target of DBS which is introduced as below.
Various educational and behavioral treatments have been the mainstay of the management of ASD. Most experts agree that the treatment for ASD should be individualized. Treatment of disabling symptoms such as aggression, agitation, hyperactivity, inattention, irritability, repetitive and self-injurious behavior may allow educational and behavioral interventions to proceed more effectively [ 109 ].
Increasing interest is being shown in the role of various pharmacological treatments. Medical management includes typical antipsychotics, atypical antipsychotics, antidepressants, selective serotonin reuptake inhibitors, α2-adrenergic agonists, β-adrenergic antagonist, mood stabilizers, and anticonvulsants [ 110 , 111 ]. So far, there has been no agent which has been proved effective in social communication [ 112 ]. A major factor in the choice of pharmacologic treatment is awareness of specific individual physical, behavioral or psychiatric conditions comorbid with ASD, such as obsessive-compulsive disorder, schizophrenia, mood disorder, and intellectual disability [ 113 ]. Antidepressants were the most commonly used agents followed by stimulants and antipsychotics. The high prevalence of comorbidities is reflected in the rates of psychotropic medication use in people with ASD. Antipsychotics were effective in treating the repetitive behaviors in children with ASD; however, there was not sufficient evidence on the efficacy and safety in adolescents and adults [ 114 ]. There are also alternative options including opiate antagonist, immunotherapy, hormonal agents, megavitamins and other dietary supplements [ 109 , 113 ].
However, the autistic symptoms remain refractory to medication therapy in some patients [ 115 ]. These individuals have severely progressed disease and multiple comorbidities causing decreased quality of life [ 44 , 110 ]. Interventional therapy such as deep brain stimulation (DBS) may be an alternative therapeutic option for these patients.
Two kinds of interventions have been used for treating ASD; focused intervention practices and comprehensive treatments [ 116 ]. The focused intervention practices include prompting, reinforcement, discrete trial teaching, social stories, or peer-mediated interventions. These are designed to produce specific behavioral or developmental outcomes for individual children with ASD, and used for a limited time period with the intent of demonstrating a change in the targeted behaviors. The comprehensive treatment models are a set of practices performed over an extended period of time and are intense in their application, and usually have multiple components [ 116 ].
Since it was approved by the FDA in 1997, DBS has been used to send electrical impulses to specific parts of the brain [ 117 , 118 ]. In recent years, the spectrum for which therapeutic benefit is provided by DBS has widely been expanded from movement disorders such as Parkinson's disease, essential tremor, and dystonia to psychiatric disorders. Some authors have demonstrated the efficacy of DBS for psychiatric disorders including refractory obsessive-compulsive disorder, depression, Tourette syndrome, and others for the past few years [ 119 , 120 , 121 ].
To the best of our knowledge, there have been 2 published articles of 3 patients who underwent DBS for ASD accompanied by life-threatening self-injurious behaviors not alleviated by antipsychotic medication [ 122 , 123 ]. The targets were anterior limb of the internal capsule and globus pallidus internus, only globus pallidus, and BL nucleus of the amygdala, respectively. All patients obtained some benefit from DBS. Although the first patient showed gradual re-deterioration after temporary improvement, the patient who underwent DBS of the BL nucleus experienced substantial improvement in self-injurious behavior and social communication. These experiences suggested the possibility of DBS for the treatment of ASD. For patients who did not obtain benefit from other treatments, DBS may be a viable therapeutic option. Understanding the structures which contribute to the occurrence of ASD might open a new horizon for management of ASD, particularly DBS. Accompanying development of neuroimaging technique enables more accurate targeting and heightens the efficacy of DBS. However, the optimal DBS target and stimulation parameters are still unknown, and prospective controlled trials of DBS for various possible targets are required to determine optimal target and stimulation parameters for the safety and efficacy of DBS.
ASD should be considered as a complex disorder. It has many etiologies involving genetic and environmental factors, and further evidence for the role of amygdala and NA in the pathophysiology of ASD has been obtained from numerous studies. However, the key architecture of ASD development which could be a target for treatment is still an uncharted territory. Further work is needed to broaden the horizons on the understanding of ASD.
Acknowledgements
This study was partly supported by the Korea Institute of Planning & Evaluation for Technology in Food, Agriculture, Forestry, and Fisheries, Republic of Korea (311011-05-3-SB020), by the Korea Healthcare Technology R&D Project (HI11C21100200) funded by Ministry of Health & Welfare, Republic of Korea, by the Technology Innovation Program (10050154, Business Model Development for Personalized Medicine Based on Integrated Genome and Clinical Information) funded by the Ministry of Trade, Industry & Energy (MI, Korea), and by the Bio & Medical Technology Development Program of the NRF funded by the Korean government, MSIP (2015M3C7A1028926).
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 16 January 2020
Autism spectrum disorder
- Catherine Lord 1 ,
- Traolach S. Brugha 2 ,
- Tony Charman 3 ,
- James Cusack 4 ,
- Guillaume Dumas 5 ,
- Thomas Frazier 6 ,
- Emily J. H. Jones 7 ,
- Rebecca M. Jones 8 , 9 ,
- Andrew Pickles 3 ,
- Matthew W. State 10 ,
- Julie Lounds Taylor 11 &
- Jeremy Veenstra-VanderWeele 12
Nature Reviews Disease Primers volume 6 , Article number: 5 ( 2020 ) Cite this article
48k Accesses
719 Citations
423 Altmetric
Metrics details
- Autism spectrum disorders
- Cognitive neuroscience
- Paediatrics
Autism spectrum disorder is a construct used to describe individuals with a specific combination of impairments in social communication and repetitive behaviours, highly restricted interests and/or sensory behaviours beginning early in life. The worldwide prevalence of autism is just under 1%, but estimates are higher in high-income countries. Although gross brain pathology is not characteristic of autism, subtle anatomical and functional differences have been observed in post-mortem, neuroimaging and electrophysiological studies. Initially, it was hoped that accurate measurement of behavioural phenotypes would lead to specific genetic subtypes, but genetic findings have mainly applied to heterogeneous groups that are not specific to autism. Psychosocial interventions in children can improve specific behaviours, such as joint attention, language and social engagement, that may affect further development and could reduce symptom severity. However, further research is necessary to identify the long-term needs of people with autism, and treatments and the mechanisms behind them that could result in improved independence and quality of life over time. Families are often the major source of support for people with autism throughout much of life and need to be considered, along with the perspectives of autistic individuals, in both research and practice.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
111,21 € per year
only 111,21 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
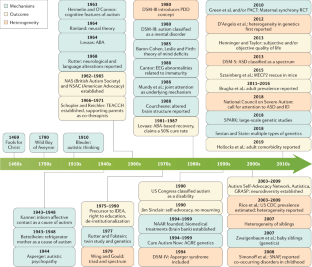
Similar content being viewed by others

Neurogenetic disorders across the lifespan: from aberrant development to degeneration
Neuroimaging genetics approaches to identify new biomarkers for the early diagnosis of autism spectrum disorder
Symptoms of autism in Williams syndrome: a transdiagnostic approach
Lord, C. et al. Autism from 2 to 9 years of age. Arch. Gen. Psychiatry 63 , 694–701 (2006). This paper establishes that autism is a stable diagnosis (as a spectrum) beginning at least by 2 years of age. The paper also establishes parent interview and clinician observation as predictive of autism at 9 years of age. Finally, it is the first paper that shows that the specific DSM-IV-TR diagnoses is unstable across childhood but that the instability is almost all shifting across categories not outside the spectrum.
Article PubMed Google Scholar
Risi, S. et al. Combining information from multiple sources in the diagnosis of autism spectrum disorders. J. Am. Acad. Child Adolesc. Psychiatry 45 , 1094–1103 (2006).
Loomes, R., Hull, L. & Mandy, W. P. L. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J. Am. Acad. Child Adolesc. Psychiatry 56 , 466–474 (2017).
Brugha, T. S. et al. Epidemiology of autism in adults across age groups and ability levels. Br. J. Psychiatry 209 , 498–503 (2016). This paper uses active case-finding to provide representative estimates of the prevalence of autism and demonstrated that rates of autism in men and women are equivalent in adults with moderate-to-profound intellectual disability.
Brugha, T., Bankart, J., McManus, S. & Gullon-Scott, F. CDC autism rate: misplaced reliance on passive sampling? Lancet 392 , 732–733 (2018).
Baxter, A. J. et al. The epidemiology and global burden of autism spectrum disorders. Psychol. Med. 45 , 601–613 (2015).
Article CAS PubMed Google Scholar
Elsabbagh, M. et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 5 , 160–179 (2012).
Article PubMed PubMed Central Google Scholar
Magnusson, C. et al. Migration and autism spectrum disorder: population-based study. Br. J. Psychiatry 201 , 109–115 (2012).
Goodman, R. & Richards, H. Child and adolescent psychiatric presentations of second-generation Afro-Caribbeans in Britain. Br. J. Psychiatry 167 , 362–369 (1995).
Dyches, T. T., Wilder, L. K., Sudweeks, R. R., Obiakor, F. E. & Algozzine, B. Multicultural issues in autism. J. Autism Dev. Disord. 34 , 211–222 (2004).
Keen, D. V., Reid, F. D. & Arnone, D. Autism, ethnicity and maternal immigration. Br. J. Psychiatry 196 , 274–281 (2010).
McManus, S., Bebbington, P., Jenkins, R. & Brugha, T. Adult Psychiatric Morbidity Survey: mental health and wellbeing in England, 2014. NHS https://digital.nhs.uk/data-and-information/publications/statistical/adult-psychiatric-morbidity-survey/adult-psychiatric-morbidity-survey-survey-of-mental-health-and-wellbeing-england-2014 (2016).
GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392 , 1789–1858 (2018).
Article Google Scholar
Marcheselli, F. et al. Mental health of children and young people in England, 2017. NHS https://digital.nhs.uk/data-and-information/publications/statistical/mental-health-of-children-and-young-people-in-england/2017/2017 (2018).
Brugha, T. C. et al. Autism Spectrum Disorder, Adult Psychiatric Morbidity Survey 2014. (2014).
Lundstrom, S., Reichenberg, A., Anckarsater, H., Lichtenstein, P. & Gillberg, C. Autism phenotype versus registered diagnosis in Swedish children: prevalence trends over 10 years in general population samples. BMJ 350 , h1961 (2015).
Tromans, S., Chester, V., Kiani, R., Alexander, R. & Brugha, T. The prevalence of autism spectrum disorders in adult psychiatric inpatients: a systematic review. Clin. Pract. Epidemiol. Ment. Health 14 , 177–187 (2018).
Modabbernia, A., Velthorst, E. & Reichenberg, A. Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Mol. Autism 8 , 13 (2017).
Article PubMed PubMed Central CAS Google Scholar
Wu, S. et al. Advanced parental age and autism risk in children: a systematic review and meta-analysis. Acta Psychiatr. Scand. 135 , 29–41 (2017).
Taylor, L. E., Swerdfeger, A. L. & Eslick, G. D. Vaccines are not associated with autism: an evidence-based meta-analysis of case-control and cohort studies. Vaccine 32 , 3623–3629 (2014).
Lai, M.-C., Lombardo, M. V. & Baron-Cohen, S. Autism. Lancet 383 , 896–910 (2014).
Velikonja, T., Fett, A.-K. & Velthorst, E. Patterns of nonsocial and social cognitive functioning in adults with autism spectrum disorder: a systematic review and meta-analysis. JAMA Psychiatry 76 , 135–151 (2019).
McNally Keehn, R. H., Lincoln, A. J., Brown, M. Z. & Chavira, D. A. The coping cat program for children with anxiety and autism spectrum disorder: a pilot randomized controlled trial. J. Autism Dev. Disord. 43 , 57–67 (2013).
Jones, E. J. H., Gliga, T., Bedford, R., Charman, T. & Johnson, M. H. Developmental pathways to autism: a review of prospective studies of infants at risk. Neurosci. Biobehav. Rev. 39 , 1–33 (2014).
Ozonoff, S. et al. Recurrence risk for autism spectrum disorders: a baby siblings research consortium study. Pediatrics 128 , e488–e495 (2011).
PubMed PubMed Central Google Scholar
Jones, R. M. & Lord, C. Diagnosing autism in neurobiological research studies. Behav. Brain Res. 251 , 113–124 (2013).
Johnson, M. H. Autism: demise of the innate social orienting hypothesis. Curr. Biol. 24 , R30–R31 (2014).
Johnson, M. H., Jones, E. J. H. & Gliga, T. Brain adaptation and alternative developmental trajectories. Dev. Psychopathol. 27 , 425–442 (2015).
The Lancet Psychiatry. Of mice and mental health. Lancet Psychiatry 6 , 877 (2019).
Nelson, C. A. et al. An integrative, multidisciplinary approach to the study of brain-behavior relations in the context of typical and atypical development. Dev. Psychopathol. 14 , 499–520 (2002).
Cross-Disorder Group of the Psychiatric Genomics Consortium et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat. Genet. 45 , 984–994 (2013).
Article CAS Google Scholar
Gaugler, T. et al. Most genetic risk for autism resides with common variation. Nat. Genet. 46 , 881–885 (2014).
Article CAS PubMed PubMed Central Google Scholar
Wang, K., Gaitsch, H., Poon, H., Cox, N. J. & Rzhetsky, A. Classification of common human diseases derived from shared genetic and environmental determinants. Nat. Genet. 49 , 1319–1325 (2017).
Sanders, S. J. et al. Insights into autism spectrum disorder genomic architecture and biology from 71 risk loci. Neuron 87 , 1215–1233 (2015).
Satterstrom, F. K. et al. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Preprint at https://doi.org/10.1101/484113 (2019).
Sanders, S. J. et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 485 , 237–241 (2012).
Neale, B. M. et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 485 , 242–245 (2012).
O’Roak, B. J. et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485 , 246–250 (2012).
Sanders, S. J. et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron 70 , 863–885 (2011).
Levy, D. et al. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron 70 , 886–897 (2011).
Sebat, J. et al. Strong association of de novo copy number mutations with autism. Science 316 , 445–449 (2007). This paper is the first to focus explicitly on simplex autism and show the importance of de novo CNVs in simplex cases, versus familial cases, versus controls.
Grove, J. et al. Identification of common genetic risk variants for autism spectrum disorder. Nat. Genet. 51 , 431–444 (2019).
Willsey, J. et al. De novo coding variants are strongly associated with Tourette syndrome. Eur. Neuropsychopharmacol. 29 , S737 (2019).
Epi4K Consortium. Epi4K: gene discovery in 4,000 genomes. Epilepsia 53 , 1457–1467 (2012).
Jamain, S. et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet. 34 , 27–29 (2003). This is the first paper to show a de novo loss-of-function mutation in a synaptic gene associated with non-syndromic autism and was a harbinger for many of the findings that came after.
Iossifov, I. et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature 515 , 216–221 (2014).
De Rubeis, S. et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 515 , 209–215 (2014).
Sestan, N. & State, M. W. Lost in translation: traversing the complex path from genomics to therapeutics in autism spectrum disorder. Neuron 100 , 406–423 (2018).
State, M. W. & Sestan, N. The emerging biology of autism spectrum disorders. Science 337 , 1301–1303 (2012).
Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511 , 421–427 (2014).
Article PubMed Central CAS Google Scholar
Devlin, B. & Scherer, S. W. Genetic architecture in autism spectrum disorder. Curr. Opin. Genet. Dev. 22 , 229–237 (2012).
de la Torre-Ubieta, L., Won, H., Stein, J. L. & Geschwind, D. H. Advancing the understanding of autism disease mechanisms through genetics. Nat. Med. 22 , 345–361 (2016).
SFARI Gene Website. https://gene.sfari.org/ (2019).
Parikshak, N. N. et al. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell 155 , 1008–1021 (2013).
Ben-David, E. & Shifman, S. Combined analysis of exome sequencing points toward a major role for transcription regulation during brain development in autism. Mol. Psychiatry 18 , 1054–1056 (2013).
Willsey, A. J. et al. Coexpression networks implicate human midfetal deep cortical projection neurons in the pathogenesis of autism. Cell 155 , 997–1007 (2013).
Pinto, D. et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature 466 , 368–372 (2010).
Gilman, S. R. et al. Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron 70 , 898–907 (2011).
Fuccillo, M. V. Striatal circuits as a common node for autism pathophysiology. Front. Neurosci. 10 , 27 (2016).
Velmeshev, D. et al. Single-cell genomics identifies cell type-specific molecular changes in autism. Science 364 , 685–689 (2019).
Mendell, J. R. et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 377 , 1713–1722 (2017).
Mercuri, E. et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N. Engl. J. Med. 378 , 625–635 (2018).
Matharu, N. et al. CRISPR-mediated activation of a promoter or enhancer rescues obesity caused by haploinsufficiency. Science 363 , eaau0629 (2019).
Abudayyeh, O. O. et al. RNA targeting with CRISPR–cas13. Nature 550 , 280–284 (2017).
Power, J. D. et al. Customized head molds reduce motion during resting state fMRI scans. NeuroImage 189 , 141–149 (2019).
Solso, S. et al. Diffusion tensor imaging provides evidence of possible axonal overconnectivity in frontal lobes in autism spectrum disorder toddlers. Biol. Psychiatry 79 , 676–684 (2016).
Clements, C. C. et al. Evaluation of the social motivation hypothesis of autism: a systematic review and meta-analysis. JAMA Psychiatry 75 , 797–808 (2018).
Ecker, C. Brain anatomy and its relationship to behavior in adults with autism spectrum disorder: a multicenter magnetic resonance imaging study. Arch. Gen. Psychiatry 69 , 195–209 (2012).
Langen, M. et al. Changes in the development of striatum are involved in repetitive behavior in autism. Biol. Psychiatry 76 , 405–411 (2014).
Elsabbagh, M. & Johnson, M. H. Autism and the social brain: the first-year puzzle. Biol. Psychiatry 80 , 94–99 (2016).
Courchesne, E. et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology 57 , 245–254 (2001).
Hazlett, H. C. et al. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch. Gen. Psychiatry 62 , 1366–1376 (2005).
Wolff, J. J. et al. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am. J. Psychiatry 169 , 589–600 (2012).
Hazlett, H. C. et al. Early brain development in infants at high risk for autism spectrum disorder. Nature 542 , 348–351 (2017). This seminal paper, through careful recruitment and methodology, was the first to show significant early differences that may contribute to our understanding of developmental features in neural structure and circuits .
Wolff, J. J. et al. Neural circuitry at age 6 months associated with later repetitive behavior and sensory responsiveness in autism. Mol. Autism 8 , 8 (2017).
Emerson, R. W. et al. Functional neuroimaging of high-risk 6-month-old infants predicts a diagnosis of autism at 24 months of age. Sci. Transl. Med. 9 , eaag2882 (2017).
Smith, E. et al. Cortical thickness change in autism during early childhood: CT in early childhood ASD. Hum. Brain Mapp. 37 , 2616–2629 (2016).
Uddin, L. Q., Dajani, D. R., Voorhies, W., Bednarz, H. & Kana, R. K. Progress and roadblocks in the search for brain-based biomarkers of autism and attention-deficit/hyperactivity disorder. Transl. Psychiatry 7 , e1218 (2017).
Herringshaw, A. J., Ammons, C. J., DeRamus, T. P. & Kana, R. K. Hemispheric differences in language processing in autism spectrum disorders: a meta-analysis of neuroimaging studies. Autism Res. 9 , 1046–1057 (2016).
He, Y., Byrge, L. & Kennedy, D. P. Non-replication of functional connectivity differences in autism spectrum disorder across multiple sites and denoising strategies. Preprint at https://doi.org/10.1101/640797 (2019).
Lawrence, K. E., Hernandez, L. M., Bookheimer, S. Y. & Dapretto, M. Atypical longitudinal development of functional connectivity in adolescents with autism spectrum disorder. Autism Res. 12 , 53–65 (2019).
Plitt, M., Barnes, K. A., Wallace, G. L., Kenworthy, L. & Martin, A. Resting-state functional connectivity predicts longitudinal change in autistic traits and adaptive functioning in autism. Proc. Natl Acad. Sci. USA 112 , E6699–E6706 (2015).
Di Martino, A. et al. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol. Psychiatry 19 , 659–667 (2014).
Doyle-Thomas, K. A. R. et al. Atypical functional brain connectivity during rest in autism spectrum disorders. Ann. Neurol. 77 , 866–876 (2015).
Supekar, K. et al. Brain hyperconnectivity in children with autism and its links to social deficits. Cell Rep. 5 , 738–747 (2013).
Dajani, D. R. & Uddin, L. Q. Local brain connectivity across development in autism spectrum disorder: a cross-sectional investigation. Autism Res. 9 , 43–54 (2016).
Hull, J. V. et al. Resting-state functional connectivity in autism spectrum disorders: a review. Front. Psychiatry 7 , 205 (2017).
Lombardo, M. V. et al. Different functional neural substrates for good and poor language outcome in autism. Neuron 86 , 567–577 (2015).
Carlisi, C. O. et al. Disorder-specific and shared brain abnormalities during vigilance in autism and obsessive-compulsive disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2 , 644–654 (2017).
Alaerts, K., Swinnen, S. P. & Wenderoth, N. Sex differences in autism: a resting-state fMRI investigation of functional brain connectivity in males and females. Soc. Cogn. Affect. Neurosci. 11 , 1002–1016 (2016).
Kirkovski, M., Enticott, P. G., Hughes, M. E., Rossell, S. L. & Fitzgerald, P. B. Atypical neural activity in males but not females with autism spectrum disorder. J. Autism Dev. Disord. 46 , 954–963 (2016).
Venkataraman, A. et al. Pivotal response treatment prompts a functional rewiring of the brain among individuals with autism spectrum disorder. NeuroReport 27 , 1081–1085 (2016).
Levisohn, P. M. The autism-epilepsy connection. Epilepsia 48 , 33–35 (2007).
Cantor, D. S., Thatcher, R. W., Hrybyk, M. & Kaye, H. Computerized EEG analyses of autistic children. J. Autism Dev. Disord. 16 , 169–187 (1986).
Lefebvre, A. et al. Alpha waves as a neuromarker of autism spectrum disorder: the challenge of reproducibility and heterogeneity. Front. Neurosci. 12 , 662 (2018).
Tierney, A. L., Gabard-Durnam, L., Vogel-Farley, V., Tager-Flusberg, H. & Nelson, C. A. Developmental trajectories of resting EEG power: an endophenotype of autism spectrum disorder. PLOS ONE 7 , e39127 (2012).
Oberman, L. M. et al. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Cogn. Brain Res. 24 , 190–198 (2005).
Fan, Y.-T., Decety, J., Yang, C.-Y., Liu, J.-L. & Cheng, Y. Unbroken mirror neurons in autism spectrum disorders. J. Child Psychol. Psychiatry 51 , 981–988 (2010).
Southgate, V. & Hamilton, A. F. Unbroken mirrors: challenging a theory of autism. Trends Cogn. Sci. 12 , 225–229 (2008).
Bernier, R., Aaronson, B. & McPartland, J. The role of imitation in the observed heterogeneity in EEG mu rhythm in autism and typical development. Brain Cogn. 82 , 69–75 (2013).
Raymaekers, R., Wiersema, J. R. & Roeyers, H. EEG study of the mirror neuron system in children with high functioning autism. Brain Res. 1304 , 113–121 (2009).
Dumas, G., Soussignan, R., Hugueville, L., Martinerie, J. & Nadel, J. Revisiting mu suppression in autism spectrum disorder. Brain Res. 1585 , 108–119 (2014). This paper replicates the mu suppression deficits in autism during action observation but questions, through high-density spectral analyses and source reconstruction, its previously drawn relation to the mirror neuron system.
Marco, E. J., Hinkley, L. B. N., Hill, S. S. & Nagarajan, S. S. Sensory processing in autism: a review of neurophysiologic findings. Pediatr. Res. 69 , 48R–54R (2011).
Schwartz, S., Shinn-Cunningham, B. & Tager-Flusberg, H. Meta-analysis and systematic review of the literature characterizing auditory mismatch negativity in individuals with autism. Neurosci. Biobehav. Rev. 87 , 106–117 (2018).
Kang, E. et al. Atypicality of the N170 event-related potential in autism spectrum disorder: a meta-analysis. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3 , 657–666 (2018).
Bonnet-Brilhault, F. et al. GABA/glutamate synaptic pathways targeted by integrative genomic and electrophysiological explorations distinguish autism from intellectual disability. Mol. Psychiatry 21 , 411–418 (2016).
Schilbach, L. Towards a second-person neuropsychiatry. Phil. Trans. R. Soc. B 371 , 20150081 (2016). This review supports that psychiatric disorders are more commonly characterized by impairments of social interaction rather than social observation, and advocates for an interactive turn in neuropsychiatry .
Barraza, P. et al. Implementing EEG hyperscanning setups. MethodsX 6 , 428–436 (2019).
Dumas, G., de Guzman, G. C., Tognoli, E. & Kelso, J. A. The human dynamic clamp as a paradigm for social interaction. Proc. Natl Acad. Sci. USA 111 , E3726–E3734 (2014).
Jones, E. J. H. et al. Reduced engagement with social stimuli in 6-month-old infants with later autism spectrum disorder: a longitudinal prospective study of infants at high familial risk. J. Neurodev. Disord. 8 , 7 (2016).
Ciarrusta, J. et al. Social brain functional maturation in newborn infants with and without a family history of autism spectrum disorder. JAMA Netw. Open 2 , e191868 (2019).
Levin, A. R., Varcin, K. J., O’Leary, H. M., Tager-Flusberg, H. & Nelson, C. A. EEG power at 3 months in infants at high familial risk for autism. J. Neurodev. Disord. 9 , 34 (2017).
Kolesnik, A. et al. Increased cortical reactivity to repeated tones at 8 months in infants with later ASD. Transl. Psychiatry 9 , 46 (2019).
Rippon, G., Brock, J., Brown, C. & Boucher, J. Disordered connectivity in the autistic brain: challenges for the ‘new psychophysiology’. Int. J. Psychophysiol. 63 , 164–172 (2007).
Rosenberg, A., Patterson, J. S. & Angelaki, D. E. A computational perspective on autism. Proc. Natl Acad. Sci. USA 112 , 9158–9165 (2015).
Masuda, F. et al. Motor cortex excitability and inhibitory imbalance in autism spectrum disorder assessed with transcranial magnetic stimulation: a systematic review. Transl. Psychiatry 9 , 110 (2019).
O’Reilly, C., Lewis, J. D. & Elsabbagh, M. Is functional brain connectivity atypical in autism? A systematic review of EEG and MEG studies. PLOS ONE 12 , e0175870 (2017).
Khan, S. et al. Somatosensory cortex functional connectivity abnormalities in autism show opposite trends, depending on direction and spatial scale. Brain 138 , 1394–1409 (2015).
Chen, H., Nomi, J. S., Uddin, L. Q., Duan, X. & Chen, H. Intrinsic functional connectivity variance and state-specific under-connectivity in autism. Hum. Brain Mapp. 38 , 5740–5755 (2017).
Catarino, A., Churches, O., Baron-Cohen, S., Andrade, A. & Ring, H. Atypical EEG complexity in autism spectrum conditions: a multiscale entropy analysis. Clin. Neurophysiol. 122 , 2375–2383 (2011).
Engemann, D. A. et al. Robust EEG-based cross-site and cross-protocol classification of states of consciousness. Brain 141 , 3179–3192 (2018).
Open Science Collaboration. Psychology. Estimating the reproducibility of psychological science. Science 349 , aac4716 (2015).
Lord, C. et al. Autism diagnostic observation schedule: ADOS-2 (Western Psychological Services, 2012).
Regier, D. A. et al. DSM-5 field trials in the United States and Canada, part II: test-retest reliability of selected categorical diagnoses. Am. J. Psychiatry 170 , 59–70 (2013).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders , 5th Edn (American Psychiatric Association, 2013).
World Health Organization. International classification of diseases for mortality and morbidity statistics (11th Revision). https://icd.who.int/browse11/l-m/en (WHO, 2018).
Constantino, J. N. & Charman, T. Diagnosis of autism spectrum disorder: reconciling the syndrome, its diverse origins, and variation in expression. Lancet Neurol. 15 , 279–291 (2016).
Lord, C. A multisite study of the clinical diagnosis of different autism spectrum disorders. Arch. Gen. Psychiatry 69 , 306–313 (2012).
Miller, J. N. & Ozonoff, S. The external validity of Asperger disorder: lack of evidence from the domain of neuropsychology. J. Abnorm. Psychol. 109 , 227–238 (2000).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders , Fourth Edn (American Psychiatric Association, 1994).
Green, D., Chandler, S., Charman, T., Simonoff, E. & Baird, G. Brief report: DSM-5 sensory behaviours in children with and without an autism spectrum disorder. J. Autism Dev. Disord. 46 , 3597–3606 (2016).
Ozonoff, S. et al. Diagnosis of autism spectrum disorder after age 5 in children evaluated longitudinally since infancy. J. Am. Acad. Child Adolesc. Psychiatry 57 , 849–857.e2 (2018).
Russell, G., Steer, C. & Golding, J. Social and demographic factors that influence the diagnosis of autistic spectrum disorders. Soc. Psychiatry Psychiatr. Epidemiol. 46 , 1283–1293 (2011).
Charman, T. & Gotham, K. Measurement issues: screening and diagnostic instruments for autism spectrum disorders—lessons from research and practice. Child Adolesc. Ment. Health 18 , 52–63 (2013).
Ashwood, K. L., Buitelaar, J., Murphy, D., Spooren, W. & Charman, T. European clinical network: autism spectrum disorder assessments and patient characterisation. Eur. Child Adolesc. Psychiatry 24 , 985–995 (2015).
Rutter, M., LeCouteur, A. & Lord, C. Autism Diagnostic Interview-Revised (ADI-R). (Western Psychological Services, 2003).
Durkin, M. S. et al. Autism screening and diagnosis in low resource settings: challenges and opportunities to enhance research and services worldwide. Autism Res. 8 , 473–476 (2015). This position paper highlights the challenges to translating knowledge on better awareness, understanding, identification and diagnosis (and then treatments) from the past two decades of clinical research in high-income countries into low-income and middle-income countries.
Baird, G. et al. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: the Special Needs and Autism Project (SNAP). Lancet 368 , 210–215 (2006).
Luyster, R. et al. The autism diagnostic observation schedule — toddler module: a new module of a standardized diagnostic measure for autism spectrum disorders. J. Autism Dev. Disord. 39 , 1305–1320 (2009).
de Vries, P. J. Thinking globally to meet local needs: autism spectrum disorders in Africa and other low-resource environments. Curr. Opin. Neurol. 29 , 130–136 (2016).
Article PubMed CAS Google Scholar
Georgiades, S., Bishop, S. L. & Frazier, T. Editorial perspective: longitudinal research in autism—introducing the concept of ‘chronogeneity’. J. Child Psychol. Psychiatry 58 , 634–636 (2017).
Fountain, C., Winter, A. S. & Bearman, P. S. Six developmental trajectories characterize children with autism. Pediatrics 129 , e1112–e1120 (2012).
Kim, S. H. et al. Variability in autism symptom trajectories using repeated observations from 14 to 36 months of age. J. Am. Acad. Child Adolesc. Psychiatry 57 , 837–848.e2 (2018).
Bussu, G. et al. Latent trajectories of adaptive behaviour in infants at high and low familial risk for autism spectrum disorder. Mol. Autism 10 , 13 (2019).
Zerbi, V. et al. Dysfunctional autism risk genes cause circuit-specific connectivity deficits with distinct developmental trajectories. Cereb. Cortex 28 , 2495–2506 (2018).
Fein, D. et al. Optimal outcome in individuals with a history of autism. J. Child Psychol. Psychiatry 54 , 195–205 (2013).
Anderson, D. K., Liang, J. W. & Lord, C. Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. J. Child Psychol. Psychiatry 55 , 485–494 (2014).
Chlebowski, C., Robins, D. L., Barton, M. L. & Fein, D. Large-scale use of the modified checklist for autism in low-risk toddlers. Pediatrics 131 , e1121–e1127 (2013).
Stenberg, N. et al. Identifying children with autism spectrum disorder at 18 months in a general population sample. Paediatr. Perinat. Epidemiol. 28 , 255–262 (2014).
Pierce, K., Courchesne, E. & Bacon, E. To screen or not to screen universally for autism is not the question: why the task force got it wrong. J. Pediatr. 176 , 182–194 (2016).
Siu, A. L. et al. Screening for autism spectrum disorder in young children: US Preventive Services Task Force recommendation statement. JAMA 315 , 691–696 (2016).
Øien, R. A. et al. Clinical features of children with autism who passed 18-month screening. Pediatrics 141 , e20173596 (2018).
Sánchez-García, A. B., Galindo-Villardón, P., Nieto-Librero, A. B., Martín-Rodero, H. & Robins, D. L. Toddler screening for autism spectrum disorder: a meta-analysis of diagnostic accuracy. J. Autism Dev. Disord. 49 , 1837–1852 (2019).
Marlow, M., Servili, C. & Tomlinson, M. A review of screening tools for the identification of autism spectrum disorders and developmental delay in infants and young children: recommendations for use in low- and middle-income countries. Autism Res. 12 , 176–199 (2019).
Raza, S. et al. Brief report: evaluation of the short quantitative checklist for autism in toddlers (Q-CHAT-10) as a brief screen for autism spectrum disorder in a high-risk sibling cohort. J. Autism Dev. Disord. 49 , 2210–2218 (2019).
Charman, T. et al. Testing two screening instruments for autism spectrum disorder in UK community child health services. Dev. Med. Child Neurol. 58 , 369–375 (2016).
Brett, D., Warnell, F., McConachie, H. & Parr, J. R. Factors affecting age at ASD diagnosis in UK: no evidence that diagnosis age has decreased between 2004 and 2014. J. Autism Dev. Disord. 46 , 1974–1984 (2016).
Zuckerman, K. E., Lindly, O. J. & Sinche, B. K. Parental concerns, provider response, and timeliness of autism spectrum disorder diagnosis. J. Pediatr. 166 , 1431–1439.e1 (2015).
Boterberg, S., Charman, T., Marschik, P. B., Bölte, S. & Roeyers, H. Regression in autism spectrum disorder: a critical overview of retrospective findings and recommendations for future research. Neurosci. Biobehav. Rev. 102 , 24–55 (2019).
Pearson, N., Charman, T., Happé, F., Bolton, P. F. & McEwen, F. S. Regression in autism spectrum disorder: reconciling findings from retrospective and prospective research. Autism Res. 11 , 1602–1620 (2018).
Ozonoff, S. & Iosif, A.-M. Changing conceptualizations of regression: what prospective studies reveal about the onset of autism spectrum disorder. Neurosci. Biobehav. Rev. 100 , 296–304 (2019). Despite its potential importance as a biological marker and/or subgroup of ASD, developmental regression has remained very poorly understood. This paper outlines recent data and reconceptualization about patterns of onset (and loss) that chime with a more contemporaneous understanding of ASD as a heterogeneous condition in terms of its manifestation both within and across individuals .
Brugha, T. S. et al. Validating two survey methods for identifying cases of autism spectrum disorder among adults in the community. Psychol. Med. 42 , 647–656 (2012).
Brugha, T. S. The Psychiatry of Adult Autism and Asperger Syndrome: a Practical Guide (Oxford Univ. Press, 2018).
Epstein, J., Johnson, D. E. & Conners, C. K. Conners Adult ADHD Diagnostic Interview for DSM-IV (CAADID) (MHS, 2001).
Lai, M.-C. et al. Prevalence of co-occurring mental health diagnoses in the autism population: a systematic review and meta-analysis. Lancet Psychiatry 6 , 819–829 (2019).
Havdahl, A. & Bishop, S. Heterogeneity in prevalence of co-occurring psychiatric conditions in autism. Lancet Psychiatry 6 , 794–795 (2019).
Croen, L. A. et al. The health status of adults on the autism spectrum. Autism 19 , 814–823 (2015).
Mannion, A., Leader, G. & Healy, O. An investigation of comorbid psychological disorders, sleep problems, gastrointestinal symptoms and epilepsy in children and adolescents with autism spectrum disorder. Res. Autism Spectr. Disord. 7 , 35–42 (2013).
Soke, G. N., Maenner, M. J., Christensen, D., Kurzius-Spencer, M. & Schieve, L. A. Prevalence of co-occurring medical and behavioral conditions/symptoms among 4- and 8-year-old children with autism spectrum disorder in selected areas of the United States in 2010. J. Autism Dev. Disord. 48 , 2663–2676 (2018).
Chandler, S. et al. Emotional and behavioural problems in young children with autism spectrum disorder. Dev. Med. Child Neurol. 58 , 202–208 (2016).
Pezzimenti, F., Han, G. T., Vasa, R. A. & Gotham, K. Depression in youth with autism spectrum disorder. Child Adolesc. Psychiatr. Clin. N. Am. 28 , 397–409 (2019).
Hwang, Y. I. J., Srasuebkul, P., Foley, K. R., Arnold, S. & Trollor, J. N. Mortality and cause of death of Australians on the autism spectrum. Autism Res. 12 , 806–815 (2019).
Hirvikoski, T. et al. Premature mortality in autism spectrum disorder. Br. J. Psychiatry 208 , 232–238 (2016).
Havdahl, K. A. et al. Multidimensional influences on autism symptom measures: implications for use in etiological research. J. Am. Acad. Child Adolesc. Psychiatry 55 , 1054–1063.e3 (2016).
Nicolaidis, C. et al. Comparison of healthcare experiences in autistic and non-autistic adults: a cross-sectional online survey facilitated by an academic-community partnership. J. Gen. Intern. Med. 28 , 761–769 (2013).
Schreibman, L. et al. Naturalistic developmental behavioral interventions: empirically validated treatments for autism spectrum disorder. J. Autism Dev. Disord. 45 , 2411–2428 (2015).
Tomlinson, M. et al. Setting global research priorities for developmental disabilities, including intellectual disabilities and autism: setting research priorities for developmental disabilities. J. Intellect. Disabil. Res. 58 , 1121–1130 (2014).
Rahman, A. et al. Effectiveness of the parent-mediated intervention for children with autism spectrum disorder in South Asia in India and Pakistan (PASS): a randomised controlled trial. Lancet Psychiatry 3 , 128–136 (2016).
Lovaas, O. I. Behavioral treatment and normal educational and intellectual functioning in young autistic children. J. Consult. Clin. Psychol. 55 , 3–9 (1987).
Nevill, R. E., Lecavalier, L. & Stratis, E. A. Meta-analysis of parent-mediated interventions for young children with autism spectrum disorder. Autism 22 , 84–98 (2018).
Kasari, C. et al. Randomized controlled trial of parental responsiveness intervention for toddlers at high risk for autism. Infant Behav. Dev. 37 , 711–721 (2014).
Shire, S. Y. et al. Hybrid implementation model of community-partnered early intervention for toddlers with autism: a randomized trial. J. Child Psychol. Psychiatry 58 , 612–622 (2017).
Siller, M., Hutman, T. & Sigman, M. A parent-mediated intervention to increase responsive parental behaviors and child communication in children with ASD: a randomized clinical trial. J. Autism Dev. Disord. 43 , 540–555 (2013).
Rogers, S. J. et al. Effects of a brief early start denver model (ESDM)-based parent intervention on toddlers at risk for autism spectrum disorders: a randomized controlled trial. J. Am. Acad. Child Adolesc. Psychiatry 51 , 1052–1065 (2012).
Green, J. et al. Parent-mediated communication-focused treatment in children with autism (PACT): a randomised controlled trial. Lancet 375 , 2152–2160 (2010).
Pickles, A. et al. Parent-mediated social communication therapy for young children with autism (PACT): long-term follow-up of a randomised controlled trial. Lancet 388 , 2501–2509 (2016).
Dawson, G. et al. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics 125 , e17–e23 (2010).
Charman, T. Editorial: trials and tribulations in early autism intervention research. J. Am. Acad. Child Adolesc. Psychiatry 58 , 846–848 (2019).
Rogers, S. J. et al. A multisite randomized controlled two-phase trial of the early start denver model compared to treatment as usual. J. Am. Acad. Child Adolesc. Psychiatry 58 , 853–865 (2019).
Dawson, G. et al. Early behavioral intervention is associated with normalized brain activity in young children with autism. J. Am. Acad. Child Adolesc. Psychiatry 51 , 1150–1159 (2012).
Myers, S. M., Johnson, C. P. & The Council on Children With Disabilities. Management of children with autism spectrum disorders. Pediatrics 120 , 1162–1182 (2007).
Laugeson, E. A., Frankel, F., Gantman, A., Dillon, A. R. & Mogil, C. Evidence-based social skills training for adolescents with autism spectrum disorders: the UCLA PEERS program. J. Autism Dev. Disord. 42 , 1025–1036 (2012).
Reichow, B., Servili, C., Yasamy, M. T., Barbui, C. & Saxena, S. Non-specialist psychosocial interventions for children and adolescents with intellectual disability or lower-functioning autism spectrum disorders: a systematic review. PLOS Med. 10 , e1001572 (2013).
Brignell, A. et al. Communication interventions for autism spectrum disorder in minimally verbal children. Cochrane Database Syst. Rev. 11 , CD012324 (2018).
PubMed Google Scholar
Tarver, J. et al. Child and parent outcomes following parent interventions for child emotional and behavioral problems in autism spectrum disorders: a systematic review and meta-analysis. Autism 23 , 1630–1644 (2019).
Keefer, A. et al. Exploring relationships between negative cognitions and anxiety symptoms in youth with autism spectrum disorder. Behav. Ther. 49 , 730–740 (2018).
Bearss, K. et al. Effect of parent training vs parent education on behavioral problems in children with autism spectrum disorder: a randomized clinical trial. JAMA 313 , 1524–1533 (2015).
Da Paz, N. S. & Wallander, J. L. Interventions that target improvements in mental health for parents of children with autism spectrum disorders: a narrative review. Clin. Psychol. Rev. 51 , 1–14 (2017).
Kasari, C. et al. Children with autism spectrum disorder and social skills groups at school: a randomized trial comparing intervention approach and peer composition. J. Child Psychol. Psychiatry 57 , 171–179 (2016).
Marshall, D. et al. Social stories in mainstream schools for children with autism spectrum disorder: a feasibility randomised controlled trial. BMJ Open 6 , e011748 (2016).
Taylor, J. L. et al. A systematic review of vocational interventions for young adults with autism spectrum disorders. Pediatrics 130 , 531–538 (2012).
Pallathra, A. A., Cordero, L., Wong, K. & Brodkin, E. S. Psychosocial interventions targeting social functioning in adults on the autism spectrum: a literature review. Curr. Psychiatry Rep. 21 , 5 (2019).
White, S. W. et al. Psychosocial treatments targeting anxiety and depression in adolescents and adults on the autism spectrum: review of the latest research and recommended future directions. Curr. Psychiatry Rep. 20 , 82 (2018).
Shattuck, P. T., Wagner, M., Narendorf, S., Sterzing, P. & Hensley, M. Post-high school service use among young adults with an autism spectrum disorder. Arch. Pediatr. Adolesc. Med. 165 , 141–146 (2011).
Wehman, P. et al. Effects of an employer-based intervention on employment outcomes for youth with significant support needs due to autism. Autism 21 , 276–290 (2017).
McCracken, J. T. et al. Risperidone in children with autism and serious behavioral problems. N. Engl. J. Med. 347 , 314–321 (2002).
Owen, R. et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics 124 , 1533–1540 (2009).
McPheeters, M. L. et al. A systematic review of medical treatments for children with autism spectrum disorders. Pediatrics 127 , e1312–e1321 (2011).
Anagnostou, E. et al. Metformin for treatment of overweight induced by atypical antipsychotic medication in young people with autism spectrum disorder: a randomized clinical trial. JAMA Psychiatry 73 , 928–937 (2016).
Research Units on Pediatric Psychopharmacology Autism Network. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch. Gen. Psychiatry 62 , 1266–1274 (2005).
Handen, B. L. et al. Atomoxetine, parent training, and their combination in children with autism spectrum disorder and attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 54 , 905–915 (2015).
Scahill, L. et al. Extended-release guanfacine for hyperactivity in children with autism spectrum disorder. Am. J. Psychiatry 172 , 1197–1206 (2015).
Hollander, E. et al. A double-blind placebo-controlled trial of fluoxetine for repetitive behaviors and global severity in adult autism spectrum disorders. Am. J. Psychiatry 169 , 292–299 (2012).
King, B. H. et al. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Arch. Gen. Psychiatry 66 , 583–590 (2009).
Anagnostou, E. et al. Intranasal oxytocin in the treatment of autism spectrum disorders: a review of literature and early safety and efficacy data in youth. Brain Res. 1580 , 188–198 (2014).
Guastella, A. J. et al. The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: a randomized controlled trial. J. Child Psychol. Psychiatry 56 , 444–452 (2015).
Parker, K. J. et al. A randomized placebo-controlled pilot trial shows that intranasal vasopressin improves social deficits in children with autism. Sci. Transl. Med. 11 , eaau7356 (2019).
Bolognani, F. et al. A phase 2 clinical trial of a vasopressin V1a receptor antagonist shows improved adaptive behaviors in men with autism spectrum disorder. Sci. Transl. Med. 11 , eaat7838 (2019).
Rubenstein, J. L. R. & Merzenich, M. M. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2 , 255–267 (2003).
Veenstra-VanderWeele, J. et al. Arbaclofen in children and adolescents with autism spectrum disorder: a randomized, controlled, phase 2 trial. Neuropsychopharmacology 42 , 1390–1398 (2017).
Berry-Kravis, E. et al. Mavoglurant in fragile X syndrome: results of two randomized, double-blind, placebo-controlled trials. Sci. Transl. Med. 8 , 321ra5 (2016).
Krueger, D. A. et al. Everolimus for treatment of tuberous sclerosis complex-associated neuropsychiatric disorders. Ann. Clin. Transl. Neurol. 4 , 877–887 (2017).
Georgiades, S. & Kasari, C. Reframing optimal outcomes in autism. JAMA Pediatr. 172 , 716–717 (2018).
Bishop-Fitzpatrick, L. et al. Characterizing objective quality of life and normative outcomes in adults with autism spectrum disorder: an exploratory latent class analysis. J. Autism Dev. Disord. 46 , 2707–2719 (2016).
The WHOQOL Group. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol. Med. 28 , 551–558 (1998).
Gotham, K. et al. Characterizing the daily life, needs, and priorities of adults with autism spectrum disorder from interactive autism network data. Autism 19 , 794–804 (2015).
Taylor, J. L. & Seltzer, M. M. Employment and post-secondary educational activities for young adults with autism spectrum disorders during the transition to adulthood. J. Autism Dev. Disord. 41 , 566–574 (2011).
Orsmond, G. I., Shattuck, P. T., Cooper, B. P., Sterzing, P. R. & Anderson, K. A. Social participation among young adults with an autism spectrum disorder. J. Autism Dev. Disord. 43 , 2710–2719 (2013).
Henninger, N. A. & Taylor, J. L. Outcomes in adults with autism spectrum disorders: a historical perspective. Autism 17 , 103–116 (2013).
Howlin, P. & Moss, P. Adults with autism spectrum disorders. Can. J. Psychiatry 57 , 275–283 (2012).
Farley, M. A. et al. Twenty-year outcome for individuals with autism and average or near-average cognitive abilities. Autism Res. 2 , 109–118 (2009).
Taylor, J. L., Henninger, N. A. & Mailick, M. R. Longitudinal patterns of employment and postsecondary education for adults with autism and average-range IQ. Autism 19 , 785–793 (2015).
Lai, M.-C. et al. Quantifying and exploring camouflaging in men and women with autism. Autism 21 , 690–702 (2016).
van Heijst, B. F. & Geurts, H. M. Quality of life in autism across the lifespan: a meta-analysis. Autism 19 , 158–167 (2015).
Moss, P., Mandy, W. & Howlin, P. Child and adult factors related to quality of life in adults with autism. J. Autism Dev. Disord. 47 , 1830–1837 (2017).
Bishop-Fitzpatrick, L., Mazefsky, C. A. & Eack, S. M. The combined impact of social support and perceived stress on quality of life in adults with autism spectrum disorder and without intellectual disability. Autism 22 , 703–711 (2017).
Kamio, Y., Inada, N. & Koyama, T. A nationwide survey on quality of life and associated factors of adults with high-functioning autism spectrum disorders. Autism 17 , 15–26 (2013).
Mason, D. et al. Predictors of quality of life for autistic adults. Autism Res. 11 , 1138–1147 (2018).
Autistica. Your questions shaping future autism research. https://www.autistica.org.uk/downloads/files/Autism-Top-10-Your-Priorities-for-Autism-Research.pdf (2016).
Ontario Brain Institute. Community priorities for research on neurodevelopmental disorders. http://braininstitute.ca/img/JLA-NDD-Final-Report.pdf (2018).
den Houting, J. Neurodiversity: an insider’s perspective. Autism 23 , 271–273 (2018).
Szatmari, P. Risk and resilience in autism spectrum disorder: a missed translational opportunity? Dev. Med. Child Neurol. 60 , 225–229 (2018).
Markowitz, L. A. et al. Development and psychometric evaluation of a psychosocial quality-of-life questionnaire for individuals with autism and related developmental disorders. Autism 20 , 832–844 (2016).
Ryan, S. & Cole, K. R. From advocate to activist? Mapping the experiences of mothers of children on the autism spectrum. J. Appl. Res. Intellect. Disabil. 22 , 43–53 (2009).
McCann, D., Bull, R. & Winzenberg, T. The daily patterns of time use for parents of children with complex needs: a systematic review. J. Child Health Care 16 , 26–52 (2012).
Karst, J. S. & Van Hecke, A. V. Parent and family impact of autism spectrum disorders: a review and proposed model for intervention evaluation. Clin. Child Fam. Psychol. Rev. 15 , 247–277 (2012).
Lounds, J., Seltzer, M. M., Greenberg, J. S. & Shattuck, P. T. Transition and change in adolescents and young adults with autism: longitudinal effects on maternal well-being. Am. J. Ment. Retard. 112 , 401–417 (2007).
Burke, M. & Heller, T. Individual, parent and social-environmental correlates of caregiving experiences among parents of adults with autism spectrum disorder. J. Intellect. Disabil. Res. 60 , 401–411 (2016).
Kim, S. H., Bal, V. H. & Lord, C. Longitudinal follow-up of academic achievement in children with autism from age 2 to 18. J. Child Psychol. Psychiatry 59 , 258–267 (2017).
Lord, C., Bishop, S. & Anderson, D. Developmental trajectories as autism phenotypes. Am. J. Med. Genet. C Semin. Med. Genet. 169 , 198–208 (2015).
Global Research on Developmental Disabilities Collaborators. Developmental disabilities among children younger than 5 years in 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancel Glob. Health 6 , e1100–e1121 (2018).
Kahn, R. S. et al. Schizophrenia. Nat. Rev. Dis. Primers 1 , 15067 (2015).
Patel, V. et al. Addressing the burden of mental, neurological, and substance use disorders: key messages from Disease Control Priorities, 3rd edition. Lancet 387 , 1672–1685 (2016).
Franz, L., Chambers, N., von Isenburg, M. & de Vries, P. J. Autism spectrum disorder in sub-Saharan Africa: a comprehensive scoping review. Autism Res. 10 , 723–749 (2017).
World Health Organization. Training parents to transform children’s lives. https://www.who.int/mental_health/maternal-child/PST/en/ (WHO, 2019).
Naslund, J. A. et al. Digital innovations for global mental health: opportunities for data science, task sharing, and early intervention. Curr. Treat. Options Psychiatry https://doi.org/10.1007/s40501-019-00186-8 (2019).
Sadowsky, J., Donvan, J. & Zucker, C. In a different key: the story of autism. J. Hist. Behav. Sci. 54 , 66–67 (2018). This paper presents a different, broad overview of the changes in perspective about autism and ASD over the years .
Rutter, M., Greenfeld, D. & Lockyer, L. A five to fifteen year follow-up study of infantile psychosis. II. Social and behavioural outcome. Br. J. Psychiatry 113 , 1183–1199 (1967).
Hermelin, B. & O’Connor, N. Psychological Experiments with Autistic Children (Pergamon Press, 1970).
Rimland, B. Infantile Autism: the Syndrome and its Implications for a Neural Theory of Behaviour (Meredith Publishing Company, 1964).
Frith, U. Studies in pattern detection in normal and autistic children: I. Immediate recall of auditory sequences. J. Abnorm. Psychol. 76 , 413–420 (1970).
Folstein, S. & Rutter, M. in Autism (eds. Rutter M. & Schopler E.) 219–241 (Springer, 1978).
Mundy, P., Sigman, M. & Kasari, C. A longitudinal study of joint attention and language development in autistic children. J. Autism Dev. Disord. 20 , 115–128 (1990).
Schopler, E. & Reichler, R. J. Parents as cotherapists in the treatment of psychotic children. J. Autism Child. Schizophr. 1 , 87–102 (1971).
Sinclair, J. Don’t mourn for us. Autism Network International http://www.autreat.com/dont_mourn.html (1993).
Wing, L. & Gould, J. Severe impairments of social interaction and associated abnormalities in children: epidemiology and classification. J. Autism Dev. Disord. 9 , 11–29 (1979).
Chawner, S. et al. A genetic first approach to dissecting the heterogeneity of autism: phenotypic comparison of autism risk copy number variants. Eur. Neuropsychopharmacol. 29 (Suppl. 3), S783–S784 (2019).
Modabbernia, A., Mollon, J., Boffetta, P. & Reichenberg, A. Impaired gas exchange at birth and risk of intellectual disability and autism: a meta-analysis. J. Autism Dev. Disord. 46 , 1847–1859 (2016).
Christensen, J. et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 309 , 1696–1703 (2013).
Xie, F., Peltier, M. & Getahun, D. Is the risk of autism in younger siblings of affected children moderated by sex, race/ethnicity, or gestational age? J. Dev. Behav. Pediatr. 37 , 603–609 (2016).
Guy, A. et al. Infants born late/moderately preterm are at increased risk for a positive autism screen at 2 years of age. J. Pediatr. 166 , 269–275.e3 (2015).
Schendel, D. & Bhasin, T. K. Birth weight and gestational age characteristics of children with autism, including a comparison with other developmental disabilities. Pediatrics 121 , 1155–1164 (2008).
Windham, G. C. et al. Maternal pre-pregnancy body mass index and gestational weight gain in relation to autism spectrum disorder and other developmental disorders in offspring. Autism Res. 12 , 316–327 (2019).
Schmidt, R. J. et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am. J. Clin. Nutr. 96 , 80–89 (2012).
Conde-Agudelo, A., Rosas-Bermudez, A. & Norton, M. H. Birth spacing and risk of autism and other neurodevelopmental disabilities: a systematic review. Pediatrics 137 , e20153482 (2016).
Lyall, K. et al. The changing epidemiology of autism spectrum disorders. Annu. Rev. Public Health 38 , 81–102 (2017).
Cheslack-Postava, K., Liu, K. & Bearman, P. S. Closely spaced pregnancies are associated with increased odds of autism in California sibling births. Pediatrics 127 , 246–253 (2011).
Conti, E., Mazzotti, S., Calderoni, S., Saviozzi, I. & Guzzetta, A. Are children born after assisted reproductive technology at increased risk of autism spectrum disorders? A systematic review. Hum. Reprod. 28 , 3316–3327 (2013).
Lehti, V. et al. Autism spectrum disorders in IVF children: a national case-control study in Finland. Hum. Reprod. 28 , 812–818 (2013).
Rossignol, D. A., Genuis, S. J. & Frye, R. E. Environmental toxicants and autism spectrum disorders: a systematic review. Transl. Psychiatry 4 , e360 (2014).
Curran, E. A. et al. Research review: birth by caesarean section and development of autism spectrum disorder and attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J. Child Psychol. Psychiatry 56 , 500–508 (2015).
Chandler, S., Howlin, P., Simonoff, E., Kennedy, J. & Baird, G. Comparison of parental estimate of developmental age with measured IQ in children with neurodevelopmental disorders. Child Care Health Dev. 42 , 486–493 (2016).
Charman, T. et al. IQ in children with autism spectrum disorders: data from the Special Needs and Autism Project (SNAP). Psychol. Med. 41 , 619–627 (2011).
Sparrow, S. S., Cicchetti, D. & Balla, D. A. Vineland Adaptive Behavior Scales, 2nd Edn. https://doi.org/10.1037/t15164-000 (AGS, 2005).
Jones, R. M., Pickles, A. & Lord, C. Evaluating the quality of peer interactions in children and adolescents with autism with the Penn Interactive Peer Play Scale (PIPPS). Mol. Autism 8 , 28 (2017).
Lord, C., Elsabbagh, M., Baird, G. & Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 392 , 508–520 (2018).
Duncan, A. W. & Bishop, S. L. Understanding the gap between cognitive abilities and daily living skills in adolescents with autism spectrum disorders with average intelligence. Autism 19 , 64–72 (2015).
Download references
Acknowledgements
The authors thank J. McCauley, S. Gaspar, K. Byrne and A. Holbrook from UCLA for help with manuscript preparation. S. Tromans is thanked for his updated review of the epidemiology literature. We recognize the many investigators who contributed research that we cannot cite due to space limitations. C.L. is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHHD; R01 HD081199), the National Institute of Mental Health (NIMH; R01MH081873-01A1) and the Simons Foundation. T.S.B. is supported by grants from the Health and Social Care Information Centre, Leeds, and the National Institute for Health Research (NIHR HTA; grant ref. NIHR127337). T.C. is supported by grants from Innovative Medicines Initiative 2 (no. 777394), the Medical Research Council (MRC; grants MR/K021389/1) and the NIHR (grant 13/119/18). J.C. is funded by Autistica. G.D. is supported by the Institut Pasteur. T.F. is supported by the Autism Speaks Foundation. E.J.H.J. is supported by grants from the Economic and Social Research Council (ESRC; ES/R009368/1), the Innovative Medicines Initiative 2 (no. 777394), the MRC (MR/K021389/1) and the Simons Foundation (609081). R.M.J. acknowledges the Mortimer D. Sackler Family and the NIMH (R01MH114999). J.L.T. is supported by grants from the FAR fund and the NIMH (R34 MH104428, R03 MH 112783 and R01 MH116058). A.P. is partially supported by the Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London and the NIHR (NF-SI-0617-10120). M.W.S. is supported by the National Institutes of Health (NIH; MH106934, MH109901, MH110928, MH116487 MH102342, MH111662, MH105575 and MH115747), the Overlook International Foundation and the Simons Foundation. J.V.-V. is supported by the NIH (MH016434 and MH094604), the Simons Foundation and the New York State Psychiatric Institute. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
Author information
Authors and affiliations.
Departments of Psychiatry and School of Education, University of California, Los Angeles, Los Angeles, CA, USA
Catherine Lord
Department of Health Sciences, University of Leicester, Leicester, UK
Traolach S. Brugha
Institute of Psychiatry, Psychology & Neuroscience, King’s College London, London, UK
Tony Charman & Andrew Pickles
Autistica, London, UK
James Cusack
Institut Pasteur, UMR3571 CNRS, Université de Paris, Paris, France
Guillaume Dumas
Autism Speaks, New York, NY, USA
Thomas Frazier
Centre for Brain & Cognitive Development, University of London, London, UK
Emily J. H. Jones
The Sackler Institute for Developmental Psychobiology, New York, NY, USA
Rebecca M. Jones
The Center for Autism and the Developing Brain, White Plains, NY, USA
Department of Psychiatry, Langley Porter Psychiatric Institute and Weill Institute for Neurosciences, University of California, San Francisco, CA, USA
Matthew W. State
Department of Pediatrics and Vanderbilt Kennedy Center, Vanderbilt University Medical Center, Nashville, TN, USA
Julie Lounds Taylor
Department of Psychiatry, Columbia University, New York, NY, USA
Jeremy Veenstra-VanderWeele
You can also search for this author in PubMed Google Scholar
Contributions
All authors read and edited the full document. Introduction (C.L.), Epidemiology (T.S.B.), Mechanisms/pathophysiology (M.W.S., G.D., R.M.J., T.C. and E.J.H.J.), Diagnosis, screening and prevention (T.C., E.J.H.J. and T.S.B.), Management (T.S.B., T.C., E.J.H.J., J.L.T. and J.V.-V.), Quality of life (J.L.T., J.C. and T.F.), Outlook (C.L. and A.P.), Overview of Primer (C.L.).
Corresponding author
Correspondence to Catherine Lord .
Ethics declarations
Competing interests.
C.L. acknowledges the receipt of royalties from Western Psychological Services for the sale of the Autism Diagnostic Interview-Revised (ADIR), the Autism Diagnostic Observation Schedule (ADOS) and the Social Communication Questionnaire (SCQ). T.S.B. has received royalties from Cambridge University Press and Oxford University Press. T.C. has served as a consultant to F. Hoffmann-La Roche. and has received royalties from Guilford Publications and Sage Publications. T.F. has received federal funding research support from, acted as a consultant to, received travel support from, and/or received a speaker’s honorarium from the Brain and Behaviour Research Foundation, Bristol-Myers Squibb, the Cole Family Research Fund, EcoEos, Forest Laboratories, Ingalls Foundation, IntegraGen, Kugona LLC, the National Institutes of Health, Roche Pharma, Shire Development and the Simons Foundation. J.L.T. receives compensation from Sage Publishers for editorial work. A.P. receives royalties from Imperial College Press, Oxford University Press and Western Psychological Services. M.W.S. serves on the scientific advisory boards and has stock or stock options for Arett Pharmaceuticals and BlackThorn Therapeutics. J.V.-V. has consulted or served on an advisory board for Novartis, Roche Pharmaceuticals and SynapDx, has received research funding from Forest, Novartis, Roche Pharmaceuticals, Seaside Therapeutics, SynapDx, and has received an editorial stipend from Springer and Wiley. All other authors declare no competing interests.
Additional information
Peer review information.
Nature Reviews Disease Primers thanks S. Spence, P. Szatmari and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Lord, C., Brugha, T.S., Charman, T. et al. Autism spectrum disorder. Nat Rev Dis Primers 6 , 5 (2020). https://doi.org/10.1038/s41572-019-0138-4
Download citation
Accepted : 26 November 2019
Published : 16 January 2020
DOI : https://doi.org/10.1038/s41572-019-0138-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Spontaneous instrumental approach-avoidance learning in social contexts in autism.
- Morgan Beaurenaut
- Klara Kovarski
- Julie Grèzes
Molecular Autism (2024)
Pharmacological and non-pharmacological interventions for irritability in autism spectrum disorder: a systematic review and meta-analysis with the GRADE assessment
- Hangnyoung Choi
- Jae Han Kim
- Marco Solmi
The gut metabolite indole-3-propionic acid activates ERK1 to restore social function and hippocampal inhibitory synaptic transmission in a 16p11.2 microdeletion mouse model
- Dilong Wang
- Ningning Li
Microbiome (2024)
Effects of mini-basketball training program on social communication impairments and regional homogeneity of brain functions in preschool children with autism spectrum disorder
- Dandan Chen
BMC Sports Science, Medicine and Rehabilitation (2024)
Dietary intake and gastrointestinal symptoms are altered in children with Autism Spectrum Disorder: the relative contribution of autism-linked traits
- Saijun Huang
Nutrition Journal (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Skip to main content
- Agriculture + Food
- Arts + Society
- Business + Economy
- Engineering + Technology
- Environment + Sustainability
Health + Medicine
- Resources + Energy
- Search news
- UQ responds
Suicide rate higher for people with autism
University of Queensland-led research has found people on the autism spectrum are almost 3 times more likely to die by suicide compared to non-autistic people.
Dr Damian Santomauro from UQ’s School of Public Health and the Queensland Centre for Mental Health Research led a team which conducted a systematic review of nearly 1500 international research papers.
“We aimed to quantify the risk, mortality and burden of suicide among people on the autism spectrum,” Dr Santomauro said.
“There were several alarming findings in this study, including the fact people on the autism spectrum but without intellectual disability were more than 5 times more likely to die by suicide compared to people not on the autism spectrum.
"In 2021, the total years of life lost to the increased risk of suicide in the autistic community exceeded those lost to cocaine use, rabies or testicular cancer across the total global population.
“And almost 2 per cent of all suicide deaths globally in 2021 could have been avoided if the risk for death by suicide for autistic people was not elevated.”
Dr Santomauro said there were likely many reasons for the higher associated risk.
“People on the autism spectrum often experience bullying, social rejection, stigma and discrimination – all risk factors for depressive disorders,” he said.
“There can also be other challenges for autistic people that impact their educational progress, employment, independent living and peer relationships.”
Dr Santomauro said the findings showed a critical need for interventions and prevention strategies.
“Measures to reduce risk factors for suicide among autistic people would substantially reduce the fatal burden of suicides globally and the health burden experienced by people on the autism spectrum,” he said.
“Studies like this one are important, to get an idea of how issues impact people on the autism spectrum.
“Without these estimates there would be no gauge for policy makers or service providers on the mortality and burden of suicide for autistic people.”
The study also involved researchers from Deakin University, La Trobe University, the University of Leicester, the Institute for Health Metrics and Evaluation and the University of Washington.
The study was published in Psychiatry Research .
If you or anyone you know needs support, please contact:
- Suicide Call Back Service - 1300 659 467
- Lifeline - 13 11 14
- Aboriginal & Torres Strait Islander crisis support line 13YARN - 13 92 76
- Kids Helpline - 1800 551 800
- Beyond Blue - 1300 224 636
- Headspace - 1800 650 890
- ReachOut - au.reachout.com
- MensLine Australia - 1300 789 978
- QLife - 1800 184 527
Media contact
UQ Faculty of Medicine Communications [email protected] +61 436 368 746
- Share on Twitter
- Share on Facebook
- Google Plus
Arts + Society , Indigenous Australia , Research
Industry Collaboration , Research , Resources + Energy

Environment + Sustainability , Research , Science

Experts , Research , Sport + Recreation

Experts , Health + Medicine , Research

Health + Medicine , Research

Experts , Health + Medicine , Research , Science , Awards and Achievements

Health + Medicine , Industry Collaboration , Research

Recent Headlines
The conversation, what we know about australia’s arms exports: we’ve analysed the data, crystals hold a secret history of volcanoes – and clues about future eruptions, esta ‘bola de cristal’ sabe si un volcán entrará en erupción, new covid vaccines may be coming to australia. here’s what to know about the jn.1 shots, está pensando em tomar wegovy para perder peso veja os riscos e benefícios - e como ele difere do ozempic.
+61 7 3365 1111
Other Campuses: UQ Gatton , UQ Herston
Maps and Directions
© 2024 The University of Queensland
A Member of
Privacy & Terms of use | Feedback
Authorised by: Director, Office of Marketing and Communications
ABN : 63 942 912 684 CRICOS Provider No: 00025B
Quick Links
- Emergency Contact
Social Media
- Giving to UQ
- Faculties & Divisions
- UQ Contacts
Ph. 3365 3333

IMAGES
COMMENTS
Abstract. Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by deficits in social communication and the presence of restricted interests and repetitive behaviors. There have been recent concerns about increased prevalence, and this article seeks to elaborate on factors that may influence prevalence rates, including ...
About the journal. Research in Autism Spectrum Disorders (RASD) publishes high quality empirical articles and reviews that contribute to a better understanding of Autism Spectrum Disorders (ASD) at all levels of description; genetic, neurobiological, cognitive, and behavioral. The primary focus of the journal is to …. View full aims & scope.
Autism spectrum disorders (ASD) are a group of neurodevelopmental disorders that affect individuals' social interactions, communication skills, and behavioral patterns, with significant individual differences and complex etiology. This article reviews the definition and characteristics of ASD, epidemiological profile, early research and diagnostic history, etiological studies, advances in ...
The Quantitative Checklist for Autism in Children (Q-CHART-10) screening approach approved by Transforming autism project, UK, served as the foundation for the conduction of this research 3.
Autism spectrum disorders articles from across Nature Portfolio. Autism spectrum disorders are a group of neurodevelopmental disorders that are characterized by impaired social interaction and ...
INTRODUCTION. Autism (or autism spectrum disorders, ASD) is defined on the basis of social and communication problems and repetitive and restrictive behaviors that can vary in individuals along a continuum of severity (Lord et al., 2018).A diagnosis of autism can be made as early as 18-24 months of age; it is around this age that characteristic symptoms can be distinguished from typical ...
Introduction. Autism spectrum disorder (ASD) refers to a group of early-onset, lifelong, heterogeneous neurodevelopmental conditions with complex mechanisms of emergence ().The prevalence of ASD has increased from 1 in 69 by 2012 to 1 in 44 by 2018, as reported by the Centers for Disease Control and Prevention for 2012-2018 (2, 3).Recent research estimates the male-to-female ratio is closer ...
Autism Research is an international journal which publishes research relevant to Autism Spectrum Disorder (ASD) and closely related neurodevelopmental disorders. We focus on genetic, neurobiological, immunological, epidemiological and psychological mechanisms and how these influence developmental processes in ASD. ... theoretical papers ...
Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder with an estimated prevalence of one in 54 (1.85%; (Maenner et al., 2020)), 52 million cases worldwide and 7.7 million disability adjusted life years (Baxter et al., 2014).Although ASD can be diagnosed as early as 18 months of age (Hyman et al., 2020), the latest review indicated that, globally, the mean age at ASD ...
autism papers published to date in Molecular Psychiatry, ... Research and training in autism spectrum disorder to catalyze the next genomic and neuroscience revolutions. Mol Psychiatry. 2020 ...
Autism is a major, peer-reviewed, international journal, published 8 times a year, publishing research of direct and practical relevance to help improve the quality of life for individuals with autism or autism-related disorders. It is interdisciplinary in nature, focusing on research in many areas, including: intervention; diagnosis; training; education; translational issues related to ...
Importance Autism spectrum disorder (ASD), characterized by deficits in social communication and the presence of restricted, repetitive behaviors or interests, is a neurodevelopmental disorder affecting approximately 2.3% children aged 8 years in the US and approximately 2.2% of adults. This review summarizes evidence on the diagnosis and treatment of ASD.
A total of 120 participants took part, 54 with a validated diagnosis of an Autism Spectrum Disorder from a professional and 66 without autism (controls). The obtained sample sizes allowed to reliably observe medium-size correlations within each group with alpha set at 0.05 and accepting a power of 0.80, and medium to large effect sizes with ...
Several key issues have emerged in relation to research, clinical and sociological aspects of autism. Shifts in research focus to encompass the massive heterogeneity covered under the label and appreciation that autism rarely exists in a diagnostic vacuum have brought about new questions and challenges. Diagnostic changes, increasing moves ...
Autism spectrum disorder (ASD) is a lifelong condition characterised by social communication difficulties and repetitive behaviours (American Psychiatric Association 2013). ... The final assurance of inclusive research was the involvement in the research and paper authorship of a parent with lived experiences of educational support for a young ...
In late 2001-early 2002 we received four exciting papers with findings on the genetics of autism that were published together in our March 2002 issue, with an accompanying editorial [2,3,4,5,6 ...
Abstract. Autism is a neuropsychiatric disorder characterised by severe and sustained impairment in social interaction, deviance in communication, and patterns of behaviour and interest that are ...
IntroductionThe wide range of psychosocial interventions designed to assist people with Autism Spectrum Disorder (ASD) makes it challenging to compile and hierarchize the scientific evidence that supports the efficacy of these interventions. ... Internet survey of treatments used by parents of children with autism. Research in Developmental ...
AL-Muthanna, Iraq. Abstract. Autism is a lifelong neuro developmental condition. It is characterised by differences in behavior, social. interaction, communication, special interests and sensory ...
Abstract. Autism spectrum disorder (autism) is a heterogeneous group of neurodevelopmental conditions characterized by early childhood-onset impairments in communication and social interaction alongside restricted and repetitive behaviors and interests. This review summarizes recent developments in human genetics research in autism ...
Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder characterized by complex physiological processes. Previous research has predominantly focused on static cerebral interactions, often neglecting the brain's dynamic nature and the challenges posed by network noise. To address these gaps, we introduce the Masked Connection-based Dynamic Graph Learning Network (MCDGLN). Our approach ...
Autism spectrum disorder (ASD) is a class of neurodevelopmental conditions with a large epidemiological and societal impact worldwide. ... Our UR summarizes research evidence on the genetics of ...
Policy paper: The national strategy for autistic children, young people and adults: 2021 to 2026. Google Scholar. Dillenburger K., Jordan J., McKerr L. (2013). Autism spectrum disorder: Public awareness and attitudes. ... Research in Autism Spectrum Disorders, 46, 1-7. Crossref. Google Scholar. Hickey A., Crabtree J., Stott J. (2018 ...
The paper contains no data or original research and concludes only that MERT could be studied further as an autism therapy without risk of serious harm. "This isn't an evidence-based paper.
Abstract. Autism spectrum disorder (ASD) is a set of neurodevelopmental disorders characterized by a deficit in social behaviors and nonverbal interactions such as reduced eye contact, facial expression, and body gestures in the first 3 years of life. It is not a single disorder, and it is broadly considered to be a multi-factorial disorder ...
Autism spectrum disorder is a construct used to describe individuals with a specific combination of impairments in social communication and repetitive behaviours, highly restricted interests and ...
Dr Damian Santomauro from UQ's School of Public Health and the Queensland Centre for Mental Health Research led a team which conducted a systematic review of nearly 1500 international research papers. "We aimed to quantify the risk, mortality and burden of suicide among people on the autism spectrum," Dr Santomauro said.